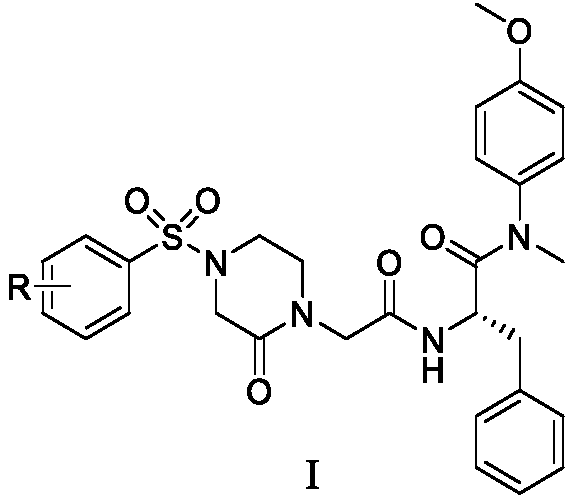
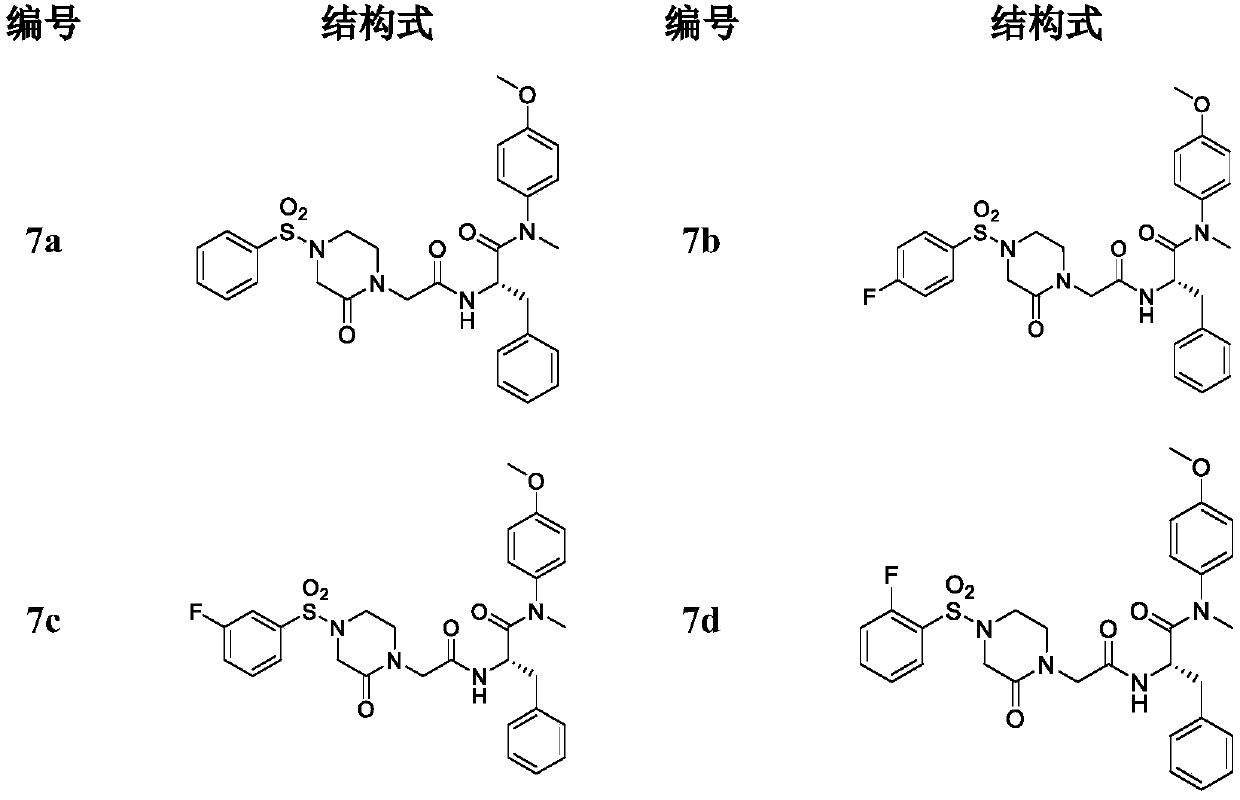
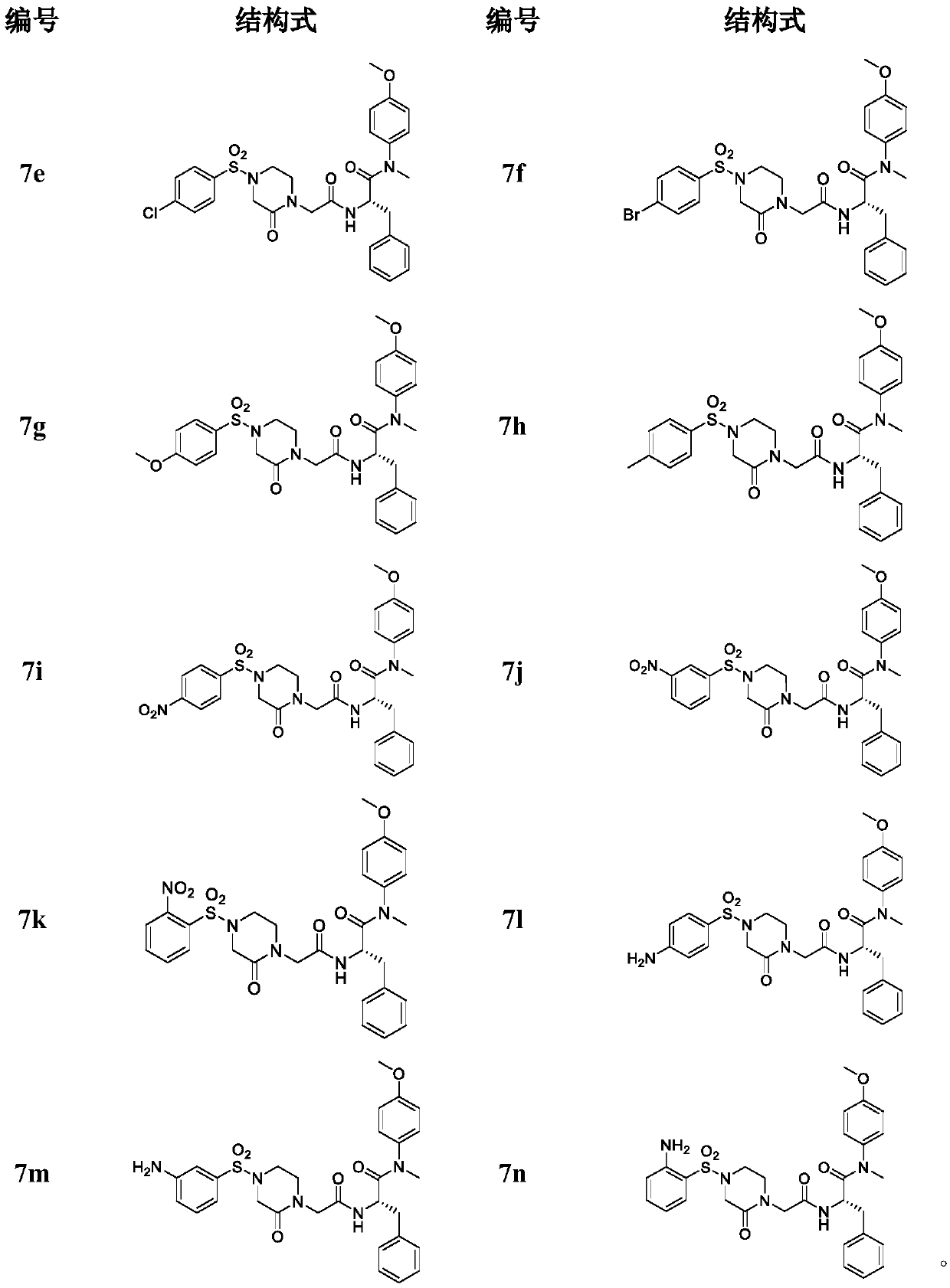
Phenylalanine derivative containing 4-(benzenesulfonyl)piperazine-2-ketone and preparing method and application of phenylalanine derivative
A technology of phenylalanine and benzenesulfonyl, which is applied in the field of organic compound synthesis and medical application, can solve the problems of low curative effect, easily induced drug resistance, poor drug-like properties, etc., and achieves the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: (S)-(1-((4-methoxyphenyl)(methyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate tert-butyl ester (2 ) preparation
[0047] Starting materials Boc-L-phenylalanine (1) (2.90g, 10.93mmol, 1.5eq.), 1H-benzotriazol-1-yloxytripyrrolidinyl hexafluorophosphate (5.69g, 10.93mmol, 1.5eq) was added to 20mL of dichloromethane, stirred in ice bath for 30min; then N,N-diisopropylethylamine (3.61mL, 21.87mmol, 3eq.) and N-methyl- 4-Aminoanisole (1.0g 7.29mmol, 1eq.), removed from the ice bath and stirred at room temperature, monitored by TLC; after 6h, the reaction was completed, the solvent was evaporated under reduced pressure, and then saturated sodium bicarbonate was added to the residue in the bottle solution 40mL, extracted with 40mL of dichloromethane, separated the organic phase, added 40mL of 1N HCl solution to wash, separated the organic phase, added 40mL of saturated sodium chloride solution to wash, dried the organic phase with anhydrous sodium sulfate, filtered...
Embodiment 2
[0052] Example 2: Preparation of (S)-2-amino-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (3)
[0053] Intermediate 2 (4.0g, 10.40mmol, 1.0eq.) was added to 30mL of dichloromethane, then trifluoroacetic acid (3.86mL, 52.02mmol, 5.0eq.) was slowly added to this solution, stirred at room temperature, monitored by TLC After 1h, the reaction was completed, then the pH of the reaction solution was adjusted to 7 with saturated sodium bicarbonate solution, 40 mL of dichloromethane was added for extraction, the organic phase was separated, washed with saturated sodium chloride solution (20 mL × 3 times), and dried over anhydrous sodium sulfate , filtered, and concentrated under reduced pressure to obtain 2.36 g of crude product of intermediate (S)-2-amino-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (3), yellow oil material with a yield of 80%.
[0054] Spectral data:
[0055] 1 H NMR (400MHz, DMSO-d 6 )δ7.29–7.13(m,3H,Ph-H),7.03–6.75(m,6H,Ph-H),3.77(s,3H,OCH 3 ),3.44–3.3...
Embodiment 3
[0058] Example 3: Preparation of intermediate (S)-2-(2-bromoacetyl)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (4)
[0059] Bromoacetic acid (117mg, 0.84mmol, 1.2eq.), O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate ( 401mg, 1.06mmol, 1.5eq.) was added to 15mL of dichloromethane, stirred in ice bath for 1h; then intermediate 3 (200mg, 0.70mmol, 1eq.) and N,N-diisopropyl Ethylamine (232μL, 1.41mmol, 2eq.), after removing the ice bath, stirred at room temperature, and monitored by TLC; after 6h, the reaction was completed, the solvent was evaporated under reduced pressure, and silica gel column chromatography (eluent EA:PE=1:4 + 2.5% triethylamine) to the intermediate (S)-2-(2-bromoacetyl)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (4) 190 mg, white oil, yield 68%.
[0060] Spectral data:
[0061] ESI-MS: m / z 405.4(M+1).C 19 h 21 BrN 2 o 3 [404.1].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com