Preparation method and application of lithium affinity-cobalt and manganese composite metal based organic frame catalyst of lithium-oxide battery cathode
A lithium-oxygen battery and composite metal technology, which is applied in battery electrodes, fuel cell-type half-cells and secondary battery-type half-cells, circuits, etc., can solve the problem of affecting the cycle life and performance of lithium-oxygen batteries. , poor electronic conductivity, etc., to achieve the effects of excellent electronic conductivity, easy operation, and improved cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
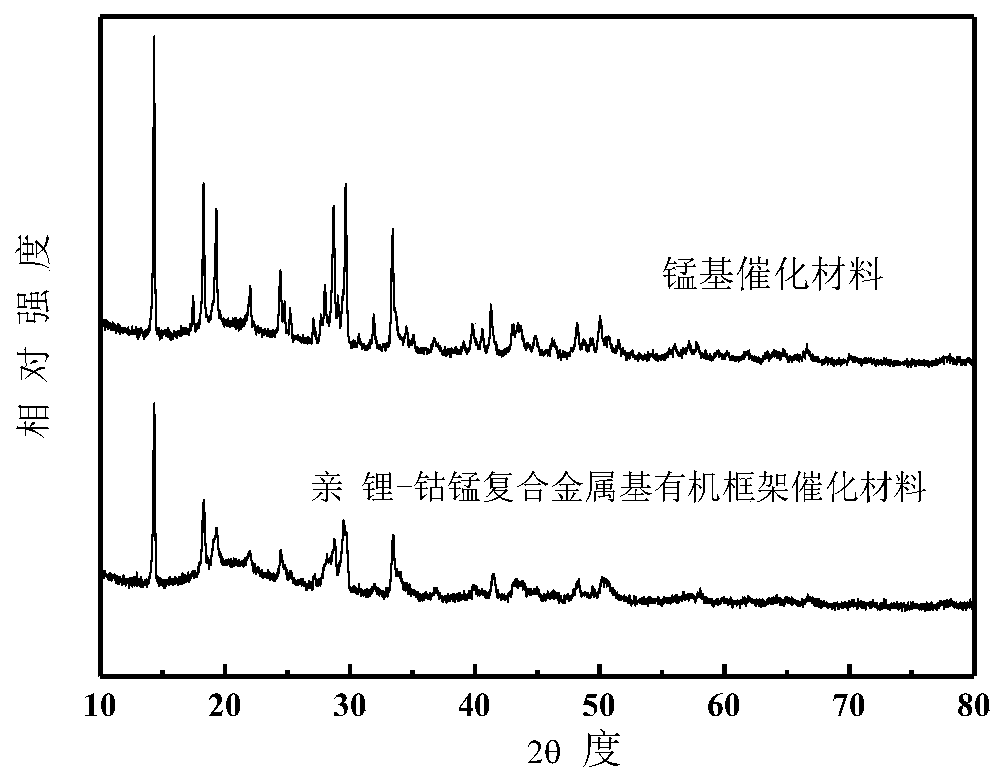
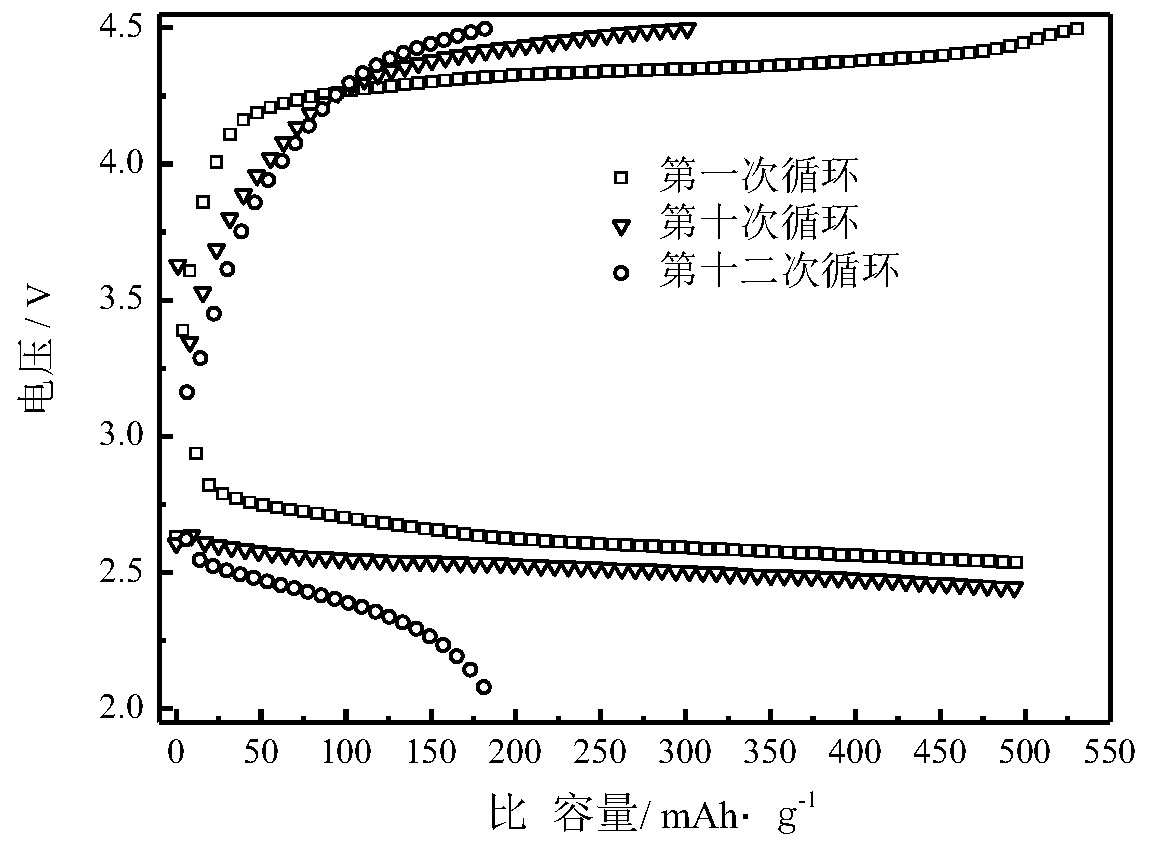
Image
Examples
Embodiment 1
[0031] (1) Add cobalt acetate, manganese acetate, and terephthalic acid to N,N-dimethylformamide at a molar ratio of 0.65:0.65:1. The ratio of N,N-dimethylformamide and cobalt acetate The molar ratio is 2:1 to prepare a mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide;
[0032] (2) Transfer the mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide to a hydrothermal kettle, and hydrothermally react at 150℃ for 40h;
[0033] (3) Add the reactant after the hydrothermal reaction to a 40% ethanol solution by mass concentration, and ultrasonically disperse for 30 min; settle the dispersed solution and discard the supernatant; repeat the above cleaning method 6 times;
[0034] (4) Centrifuge the washed reactant at 5000r / min for 10min, discard the supernatant, and keep the centrifuged product;
[0035] (5) Dry the centrifuged product at 70°C for 20 hours; after grinding through a 250-mesh sieve, a cobalt-manganese ...
Embodiment 2
[0037] (1) Add cobalt acetate, manganese acetate, and terephthalic acid to N,N-dimethylformamide at a molar ratio of 0.65:0.65:1. The ratio of N,N-dimethylformamide and cobalt acetate The molar ratio is 4:1 to prepare a mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide;
[0038] (2) Transfer the mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide to a hydrothermal kettle, and hydrothermally react at 150℃ for 40h;
[0039] (3) Add the reactant after the hydrothermal reaction to a 40% ethanol solution by mass concentration, and ultrasonically disperse for 30 min; settle the dispersed solution and discard the supernatant; repeat the above cleaning method 6 times;
[0040] (4) Centrifuge the washed reactant at 5000r / min for 10min, discard the supernatant, and keep the centrifuged product;
[0041] (5) Dry the centrifuged product at 70°C for 20 hours; after grinding through a 250-mesh sieve, a cobalt-manganese ...
Embodiment 3
[0043] (1) Add cobalt acetate, manganese acetate, and terephthalic acid to N,N-dimethylformamide at a molar ratio of 0.65:0.65:1. The ratio of N,N-dimethylformamide and cobalt acetate The molar ratio is 5:1, and the mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide is prepared;
[0044] (2) Transfer the mixed solution of cobalt acetate-manganese acetate-terephthalic acid-N,N-dimethylformamide to a hydrothermal kettle, and hydrothermally react at 150℃ for 40h;
[0045] (3) Add the reactant after the hydrothermal reaction to a 40% ethanol solution by mass concentration, and ultrasonically disperse for 30 min; settle the dispersed solution and discard the supernatant; repeat the above cleaning method 6 times;
[0046] (4) Centrifuge the washed reactant at 5000r / min for 10min, discard the supernatant, and keep the centrifuged product;
[0047] (5) Dry the centrifuged product at 70°C for 20 hours; after grinding through a 250-mesh sieve, a cobalt-ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com