Deoxynivalenol and its derivative aptamer affinity column and its preparation method and application
A technology of vomitoxin and its derivatives, applied in the direction of DNA / RNA fragments, recombinant DNA technology, etc., can solve the problems of unsatisfactory vomitoxin and its derivatives, and achieve the goal of improving pretreatment efficiency, reducing cost and time, and saving costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
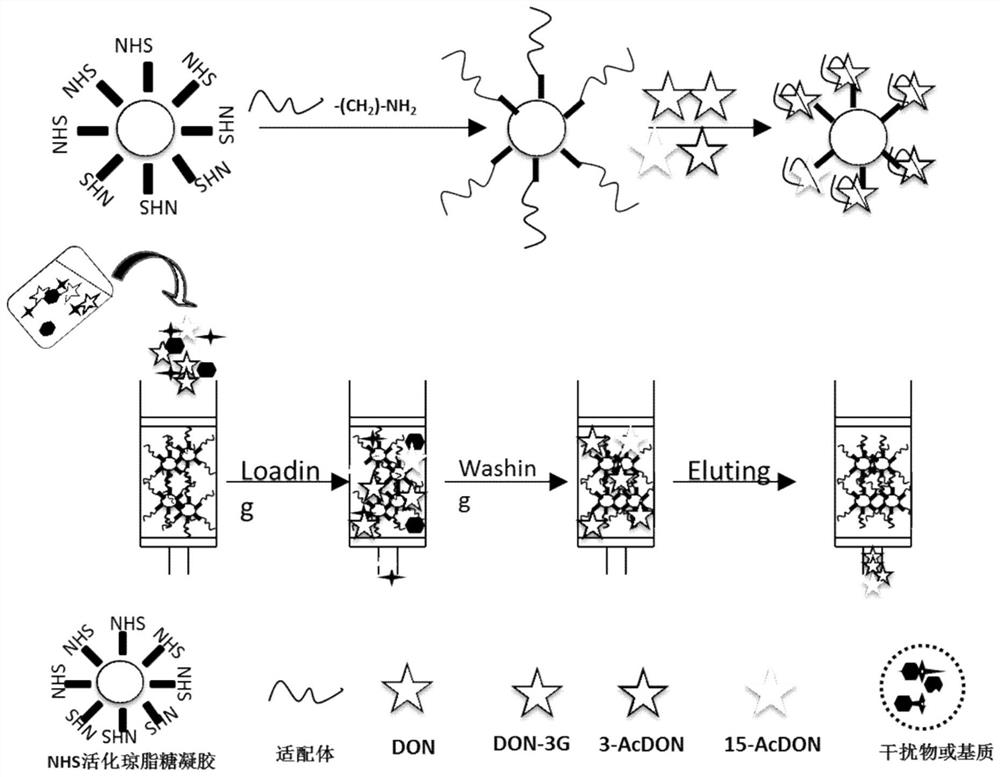
[0057] Example 1 Preparation of aptamer affinity column using amino-modified deoxynivalenol and its derivative aptamers
[0058] 1. Preparation of N-hydroxysuccinimide modified agarose. Sepharose 4B was used as the carrier and activated with N-hydroxysuccinimide.
[0059] Mix agarose with an equal volume of water in a fume hood, and add it to a reactor equipped with a pH electrode and a magnetic stirrer, and add 100 mg of N-hydroxysuccinimide per mL of agarose solution to the above agarose solution For N-hydroxysuccinimide, adjust the pH value to 11 with NaOH, control the pH value of the whole reaction at 11, control the temperature at about 20°C, and complete the reaction in 10 minutes; after the reaction, add an equal volume of ice chips to the above reaction quickly solution, and quickly poured into the Buchner funnel, with 10 times the volume of the agarose solution of cold buffer (200mM Na 2 HPO 4 , 5 mM MgCl 2 , pH 8.0) was washed by suction, and the hydroxyl groups ...
Embodiment 2
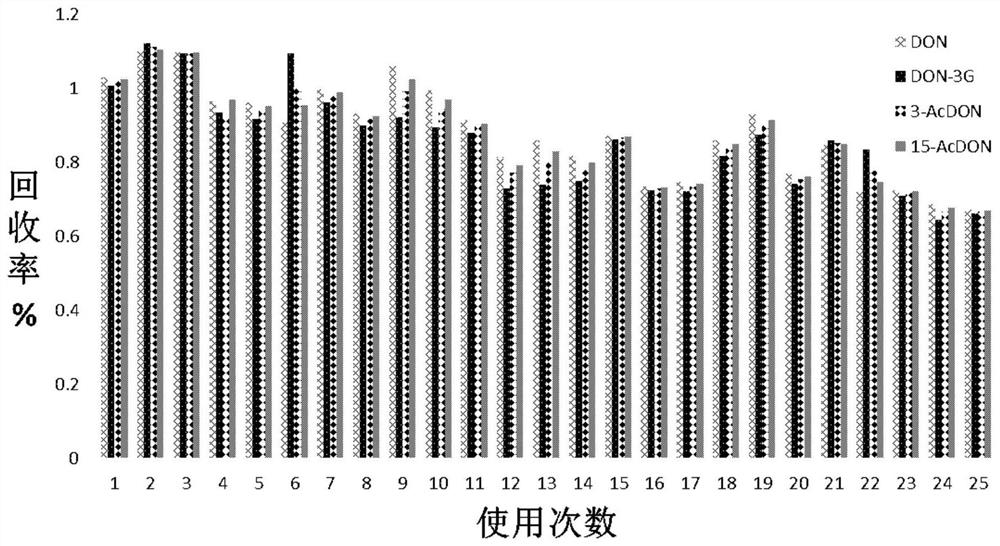
[0074] Example 2 Purification and detection of deoxynivalenol and its derivatives in wheat samples by using aptamer affinity column for deoxynivalenol and its derivatives
[0075] In this example, quantitative addition of deoxynivalenol and its derivatives standard substance to normal wheat samples, followed by purification with the aptamer affinity column of deoxynivalenol and its derivatives prepared in Example 1, followed by purification by high performance liquid chromatography- Fluorescence detector detection, determination of recovery. details as follows:
[0076] 1. Wheat sample processing
[0077] 1) Pulverize the wheat sample.
[0078] 2) According to the standards of 0.5, 5, and 50 μg per gram of sample, respectively, add deoxynivalenol and its derivative standard to the pulverized wheat sample.
[0079] 3) Weigh 5g of the sample, add 25mL of methanol-water (7:3, v / v), and place it on a homogenizer for 11000rpm high-speed homogenization for 3min.
[0080] 4) Filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com