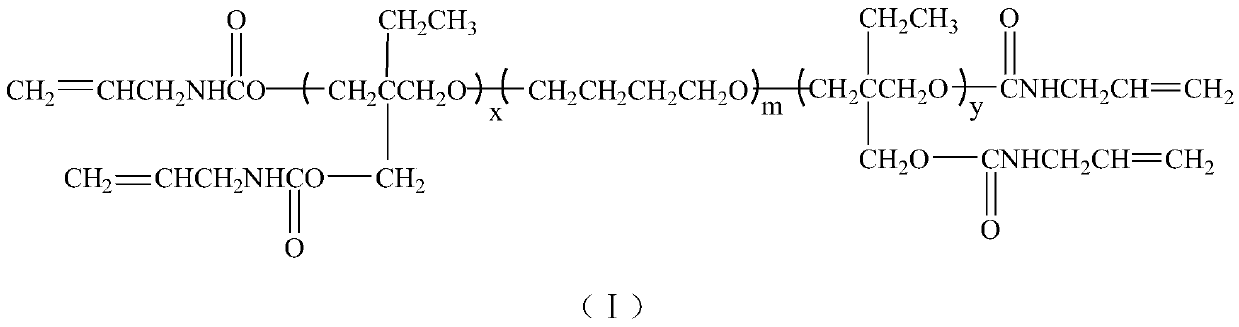Polyalkenyl polytetrahydrofuran adhesive and synthesis method thereof
A technology of polyalkenyl polytetrahydrofuran and alkenyl polytetrahydrofuran, which is applied in the direction of polyether adhesives, adhesive types, adhesives, etc., can solve the problems of harsh curing conditions, high curing temperature, and water sensitivity, and achieve improved Regularity and crosslink density, effects of high mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
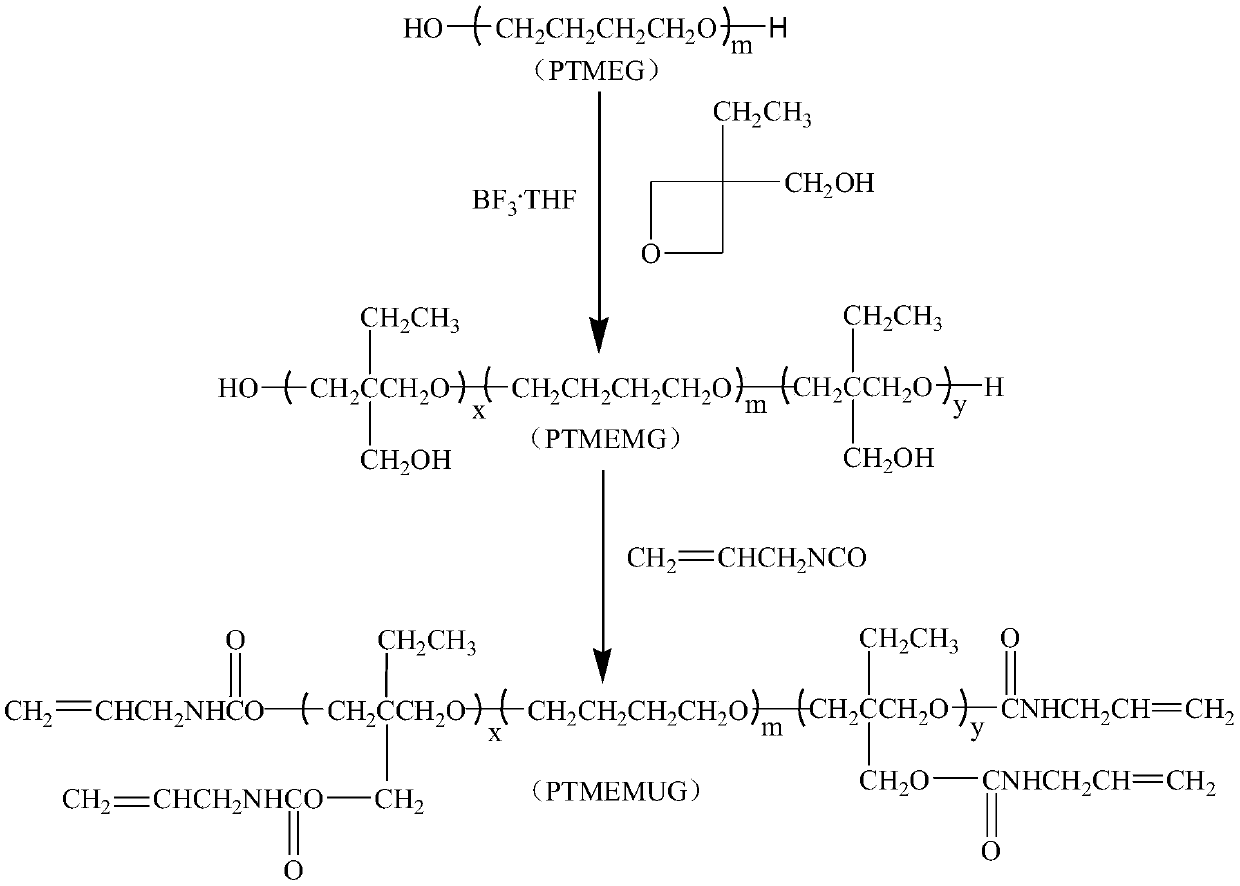
[0029] (1) Synthesis of PTMEMG
[0030] In a 250ml four-neck flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, add 50g (0.05mol) of polytetrahydrofuran diol, 100ml of 1,2-dichloroethane and 4.2g of boron trifluoride in sequence ·Tetrahydrofuran complex, stirred for 1h, cooled to 2°C, began to slowly add 11.6g of 3-hydroxymethyl-3-ethyloxetane dropwise, the dropwise time was 2h, after the dropwise addition, continue the insulation reaction 12h, after the reaction is completed, use NaHCO 3 The aqueous solution was neutralized, then washed three times with distilled water, poured into a separatory funnel, separated the organic phase, and distilled off 1,2-dichloroethane under reduced pressure to obtain 61.2 g of light yellow waxy solid.
[0031] Structural identification: IR, ν max (cm- 1 ): 3440(-OH), 2846, 2965(-CH 3 、-CH 2 -), 1106 (C-O-C).
[0032] 1 H NMR (CDCl 3 , 500MHz): 0.86, 1.65, 1.82, 3.33~3.51.
[0033] The number av...
Embodiment 2
[0041] (1) Synthesis of PTMEMG
[0042] In a 500ml four-neck flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, add 100g (0.05mol) of polytetrahydrofuran diol, 200ml of 1,2-dichloroethane and 4.5g of boron trifluoride in sequence ·Tetrahydrofuran complex, stir for 1h, cool down to 2°C, start to slowly add 11.6g of 3-hydroxymethyl-3-ethyloxetane dropwise, the dropping time is 3h, after the dropwise addition, continue to keep warm 12h, after the reaction is completed, use NaHCO 3 The aqueous solution was neutralized, then washed three times with distilled water, poured into a separatory funnel, and the organic phase was separated, and 1,2-dichloroethane was distilled off under reduced pressure to obtain 111.1 g of a pale yellow waxy solid.
[0043] The number average molecular weight was 2230, and the hydroxyl value was 98.1 mgKOH / g.
[0044] (2) Synthesis of PTMEMUG
[0045]In a three-neck flask equipped with mechanical stirring and a...
Embodiment 3
[0048] (1) Synthesis of PTMEMG
[0049] In a 500ml four-neck flask equipped with mechanical stirring, reflux condenser, thermometer and dropping funnel, add 100g (0.05mol) of polytetrahydrofuran diol, 200ml of 1,2-dichloroethane and 4.5g of boron trifluoride in sequence ·Tetrahydrofuran complex, stirred for 1h, cooled to 2°C, started to slowly add 23.2g of 3-hydroxymethyl-3-ethyloxetane dropwise, the dropwise time was 3h, after the dropwise addition, continue the heat preservation reaction 12h, after the reaction is completed, use NaHCO 3 The aqueous solution was neutralized, then washed three times with distilled water, poured into a separatory funnel, separated the organic phase, and distilled off 1,2-dichloroethane under reduced pressure to obtain 122.8 g of light yellow waxy solid.
[0050] The number average molecular weight was 2450, and the hydroxyl value was 132.8 mgKOH / g.
[0051] (2) Synthesis of PTMEMUG
[0052] In a three-neck flask equipped with mechanical stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com