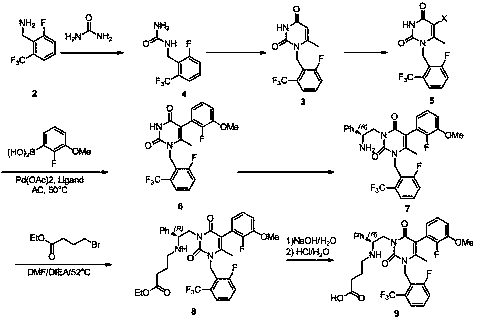
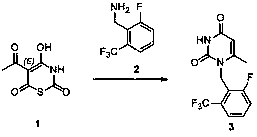
Preparation method of compound containing 6-methyluracil structure
The technology of a compound and an organic solvent is applied in the field of preparation of compounds containing 6-methyluracil structure, can solve the problems of hot flash-related bone mineral density, delayed onset of action, side effects, etc., and achieves strong impurity removal ability and simple post-processing , the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
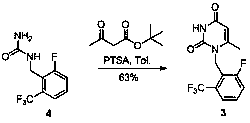
[0025]
[0026] Add 1.0 g of compound 2 and 1.07 g of compound 1 into the reaction flask, add 5 mL of DMF, heat up to 140 °C and stir for 0.5 h, HPLC monitors that the conversion of the raw materials is complete, cool down to room temperature, add 15 mL of water to quench the reaction, and a large amount of Solid, stirred for 0.5h, filtered to obtain the crude product, directly added the crude product to 1 mL of ethanol, heated to 80-90°C, continued to stir for 1h, then slowly cooled to 5-10°C, filtered, and drenched with 0.5ml of ethanol Washing, the product was a white solid with a yield of 67% and a purity of 98.6%.
[0027] The NMR data of compound 3 are as follows:
[0028] 1 H NMR (400 MHz, CDCl 3 ) δ 9.03 (s, 1H), 7.56 (d, J = 7.8 Hz, 1H), 7.46-7.41 (m, 1H), 7.30-7.25 (m, 1H), 5.61 (s, 1H), 5.38 (s, 2H), 2.17 (s, 3H).
[0029] M+H molecular ion peak 303.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com