Viologen-based ion type porous organic polymers and preparation method and application thereof
An ionic and polymer technology, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as cumbersome methods, and achieve simple equipment and low catalyst consumption The effect of less, excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of viologen-based ionic porous organic polymer V-iPOP-Cl
[0036]
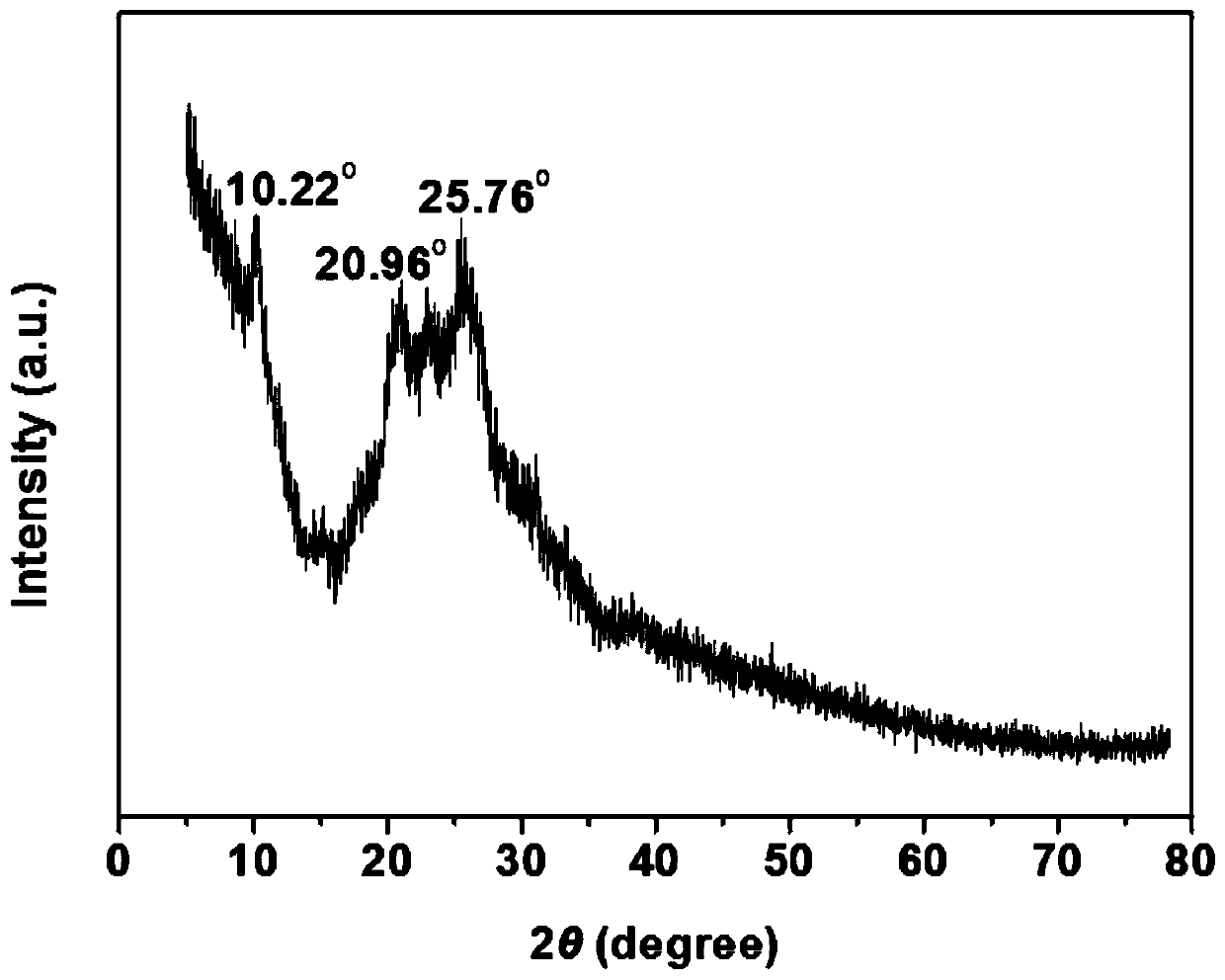
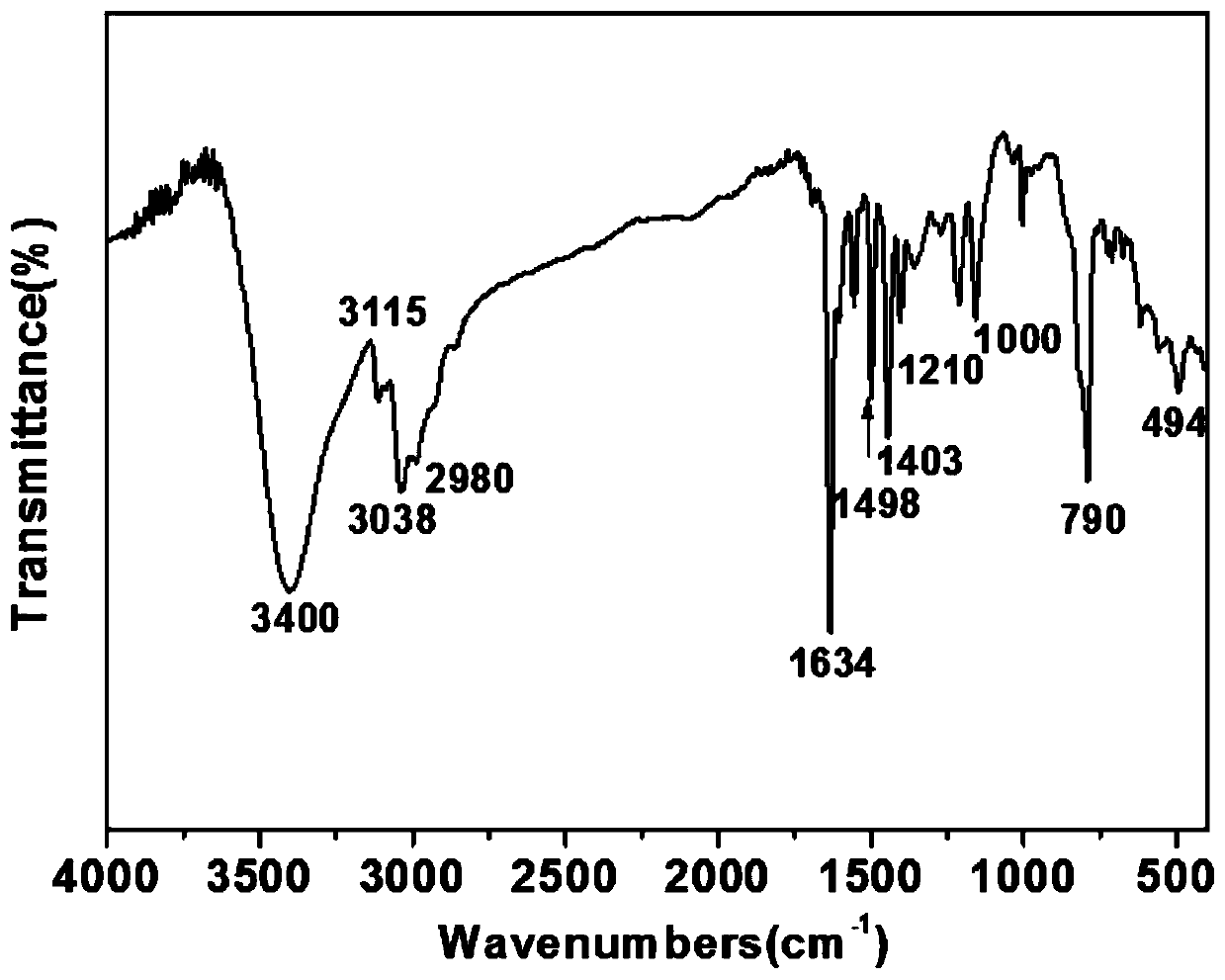
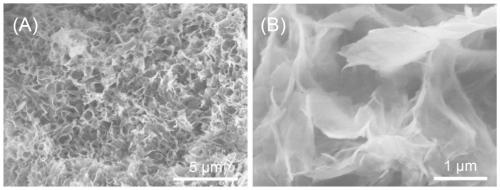
[0037] Weigh 4,4'-bipyridyl, biphenyl benzyl dichloride and add 5mL of acetonitrile respectively, stir to dissolve completely, mix the two solutions into a polytetrafluoroethylene-lined stainless steel reaction kettle, place in a 100°C oven, Reaction 48h. After the reaction was completed, the reactor was cooled to room temperature, and the solution was transferred into a beaker, then suction filtered, and washed with DMSO, deionized water and ethanol respectively. Finally, the filter cake was dried in a vacuum oven (80° C.) for 12 hours to obtain the viologen-based ionic porous organic polymer V-iPOP-Cl. The XRD spectrum of V-iPOP-Cl is as follows figure 1 As shown, the infrared IR spectrum is shown as figure 2 As shown, the SEM image is shown in image 3 As shown, V-iPOP-C under 77K N 2 The adsorption-desorption curve is as Figure 4 shown.
[0038] The viologen-based ...
Embodiment 2
[0039] Example 2: Catalytic conversion of CO by V-iPOP-Cl 2 Typical reaction optimization process and reusability
[0040] Add 2mmol epichlorohydrin and 0.05g of the catalyst V-iPOP-Cl in Example 1 into a 25mL reaction tube, stir at 40°C-80°C, and connect the CO 2 The vent tube of the gas cylinder discharges the air in the reaction tube, and then inserts a balloon (0.1MPa) filled with carbon dioxide into the reaction tube, and reacts for 48-120h. After the reaction was completed, a certain amount of ethyl acetate was added for dilution, and stirred at room temperature for 20 min. The solution was taken out, centrifuged, and the supernatant was taken out, and the yield was analyzed and calculated by gas chromatography (GC). The results are shown in Table 1. As can be seen from Table 1, V-iPOP-Cl is a very efficient heterogeneous catalyst.
[0041] Table 1 CO catalyzed by viologen-based ionic porous organic polymer V-iPOP-Cl 2 Results of cycloaddition reaction with epichloro...
Embodiment 3
[0047] In this example, the V-iPOP-Cl catalyst is used as a substrate to carry out CO 2 Research on the expansion of substrates in cycloaddition catalytic reactions, epoxy compounds are
[0048]
[0049] Wherein, R is chloromethyl, bromomethyl, phenyl, benzyloxy, n-butyl, n-hexyl or n-decyl. The experimental results are shown in Table 3.
[0050] Table 3 CO catalyzed by viologen-based ionic porous organic polymer V-iPOP-Cl 2 Cycloaddition reaction performance with different epoxy compounds
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com