Preparation method of low-cost lithium titanate material
A lithium titanate, low-cost technology, applied in chemical instruments and methods, titanium compounds, inorganic chemistry, etc., can solve the problem of high price of lithium titanate materials, and achieve superior electrochemical performance, low raw material cost, and less time-consuming. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
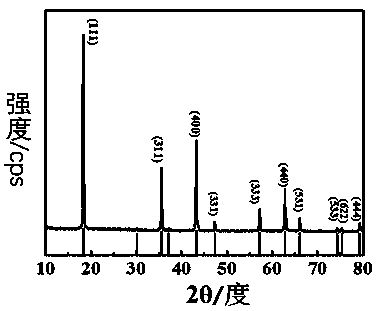
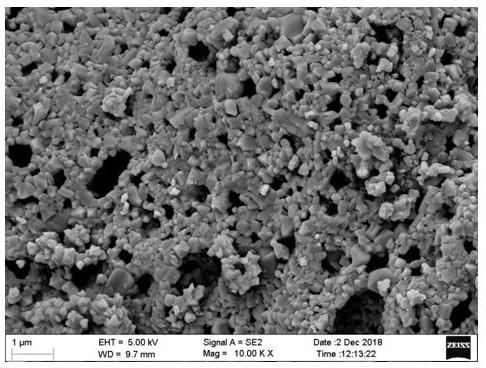
Image
Examples
Embodiment 1
[0024] (1) Preparation of peroxo-titanium complex solution
[0025] According to the mass volume ratio of 1:10, metatitanic acid and hydrogen peroxide were accurately weighed and put into the reactor for stirring, and an appropriate amount of ammonia water was added to control the pH value of the solution to be 10 to obtain a stable peroxotitanium complex solution.
[0026] (2) Preparation of lithium titanate material
[0027] Accurately weigh lithium hydroxide monohydrate according to the lithium-titanium molar ratio of 0.86 and add it to the above titanium peroxo complex solution, add an appropriate amount of dispersant polyvinylpyrrolidone, stir to form a uniform solution, freeze-dry, and calcinate at 800°C for 8 hours to obtain titanic acid lithium material.
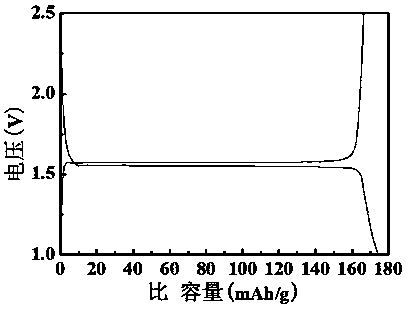
[0028] After the electrochemical performance test, after the lithium titanate material prepared in this example is prepared into a half-cell, the test shows that the first discharge specific capacity at a rate of 0....
Embodiment 2
[0030] (1) Preparation of peroxo-titanium complex solution
[0031] According to the mass volume ratio of 1:20, metatitanic acid and hydrogen peroxide were accurately weighed and put into the reactor for stirring, and an appropriate amount of ammonia water was added to control the pH value of the solution to 11 to obtain a stable peroxotitanium complex solution.
[0032] (2) Preparation of lithium titanate material
[0033] Accurately weigh lithium carbonate according to the lithium-titanium molar ratio of 0.86 and add it to the above titanium peroxo complex solution, add an appropriate amount of dispersant polyethylene glycol, stir to form a uniform solution, freeze-dry, and calcinate at 700°C for 10 hours to obtain lithium titanate material .
[0034] After the electrochemical performance test, after the lithium titanate material prepared in this example is prepared into a half-cell, the test shows that the first discharge specific capacity at a rate of 0.2C is 170mAh / g.
Embodiment 3
[0036] (1) Preparation of peroxo-titanium complex solution
[0037] According to the mass volume ratio of 1:30, metatitanic acid and hydrogen peroxide were accurately weighed and put into the reactor for stirring, and an appropriate amount of ammonia water was added to control the pH value of the solution to be 12 to obtain a stable peroxotitanium complex solution.
[0038] (2) Preparation of lithium titanate material
[0039] Accurately weigh lithium hydroxide monohydrate according to the lithium-titanium molar ratio of 0.84 and add it to the above titanium peroxo complex solution, add an appropriate amount of dispersant polyvinylpyrrolidone, stir to form a uniform solution, freeze-dry, and calcinate at 800°C for 8 hours to obtain titanic acid lithium material.
[0040] After the electrochemical performance test, after the lithium titanate material prepared in this example is prepared into a half-cell, the test shows that the first discharge specific capacity at 0.2C rate is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com