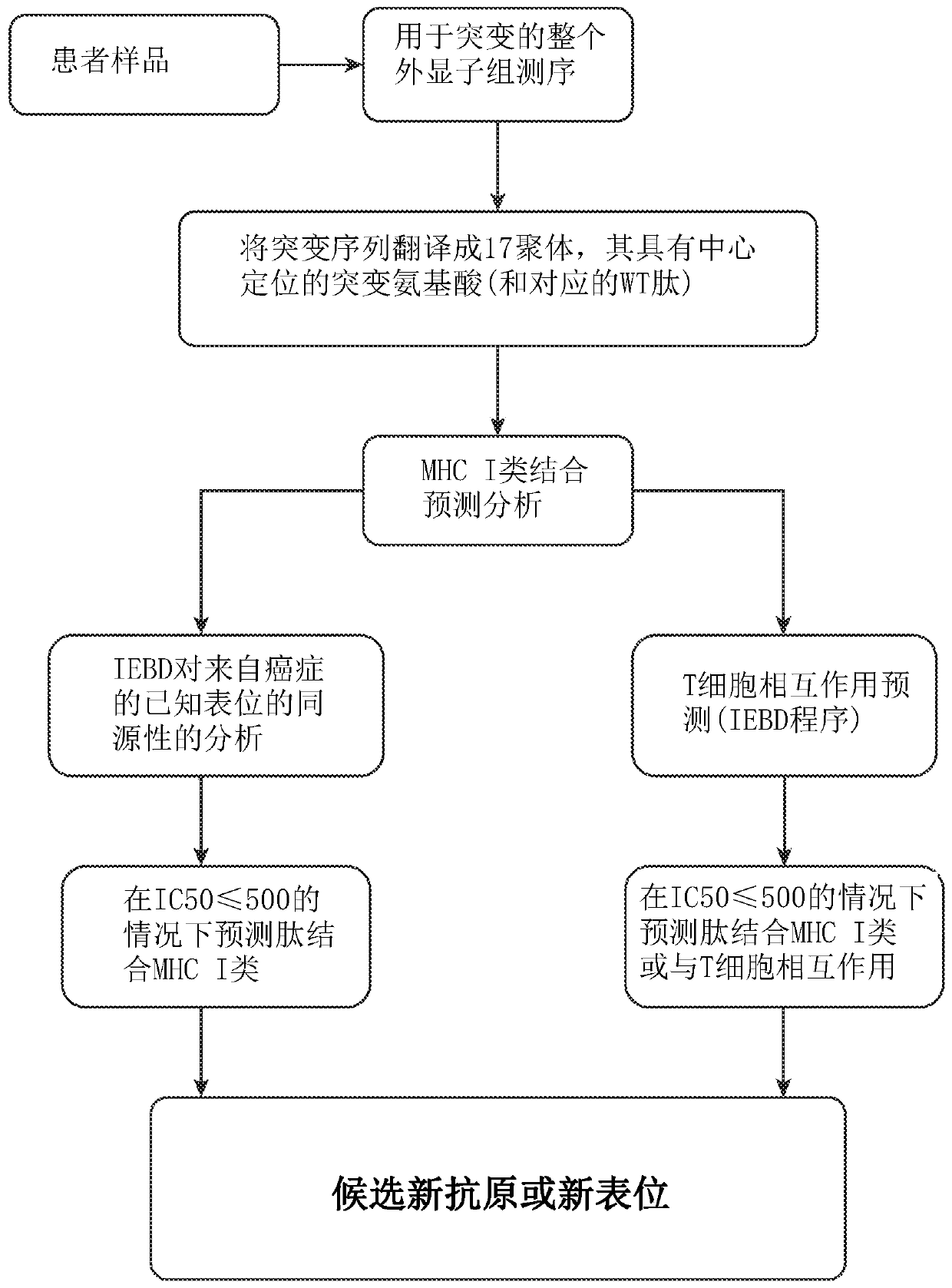
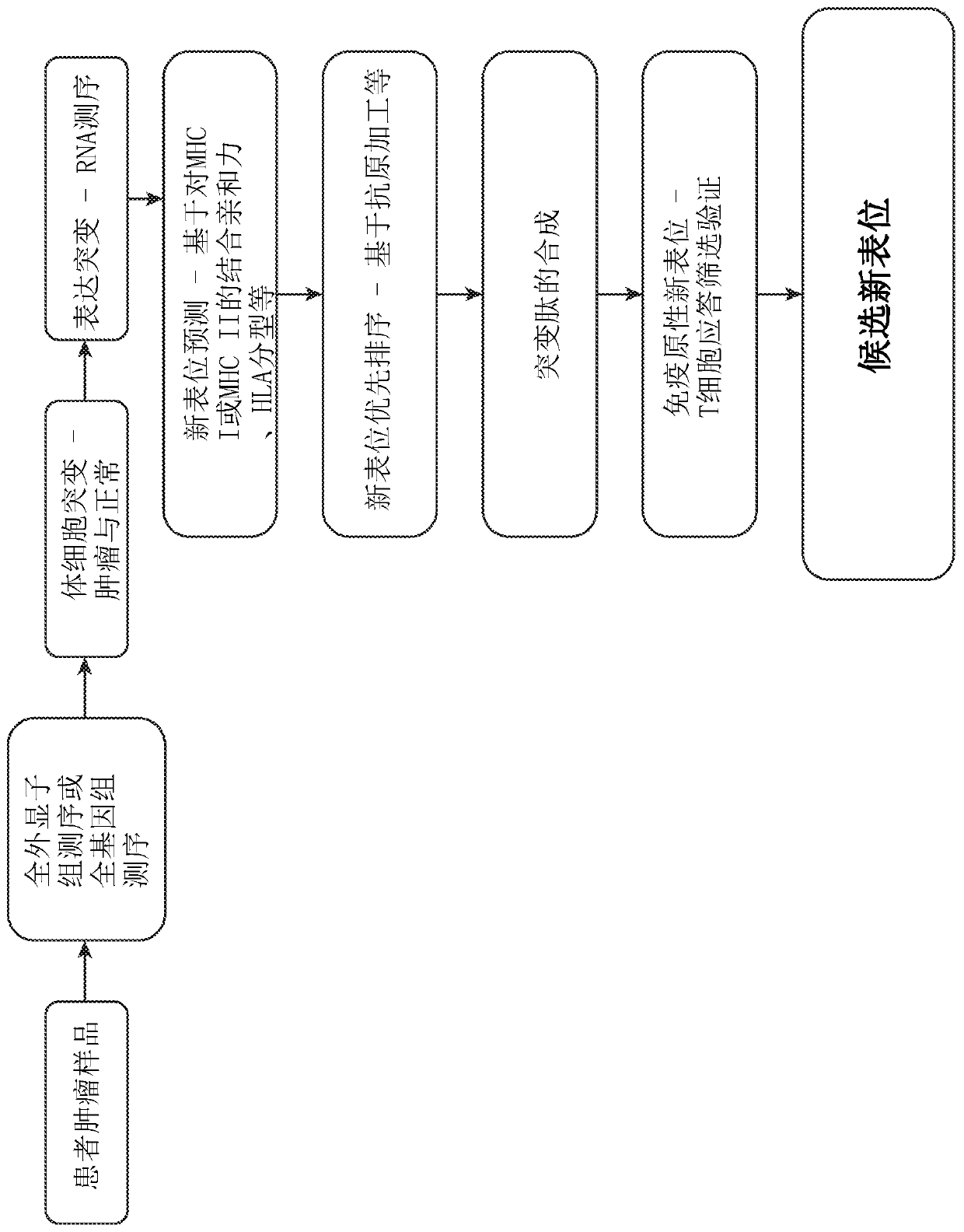
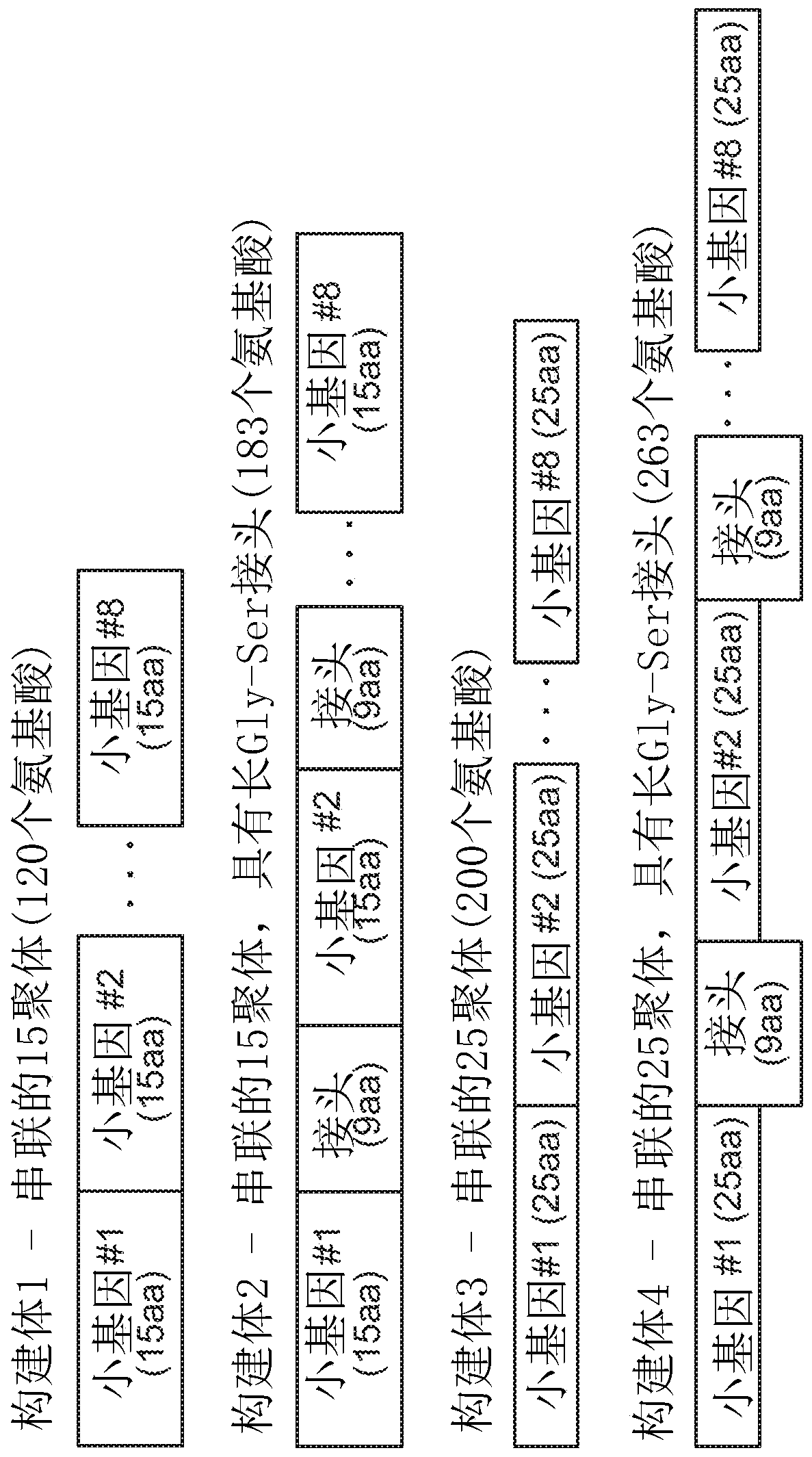
Neoepitope vaccine compositions and methods of use thereof
A composition and fusion partner technology, applied in biochemical equipment and methods, drug combinations, chemical instruments and methods, etc., can solve the problem of not being able to trigger therapeutic immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0497] Multiple neo-epitopes for insertion of Ad5 [E1-, E2b-]
[0498] Construction of vectors (Alkbh6.2, Slit3 and Atxn10.1)
[0499] Construction of Ad5[E1-, E2b-] vector: the about 20kb Xba-BamHI subfragment of pBHG11 (Bett et al., 1994, Institute of Microbiology, Toronto, Ontario, Canada (1994, Microbix, Toronto, Ontario, Canada)) Subcloning into pBluescriptKSII+ (Stratagene, La Jolla, Calif.) generated pAXB. Plasmid pAXB was digested with BspEI, end-stuffed with T4 DNA polymerase, and digested with BamHI to isolate a fragment of approximately 9.0 kb. Plasmid pAXB was also digested with BspHI, T4 DNA polymerase endfill, and BamHI digested, and the approximately 13.7 kb fragment was ligated to a previously isolated 9.0 kb fragment to generate pAXB-Δpol.
[0500] This subcloning strategy deleted a 608 bp (Δpol; Ad5 nucleotide 7274 to nucleotide 7881 ) at the amino terminus of the polymerase gene. This deletion also effectively removed the open reading frame 9.4 present on...
Embodiment 2
[0504] Multiple injections of Ad5[E1-,E2b-]-vectors containing Alkbh6.2, Slit3, and Atxn10.1 generate cell-mediated immune responses against these neoepitopes
[0505] Mice were immunized twice with mutated peptides Alkbh6.2, Slit3 and Atxn10.1 or an empty vector control, two weeks apart. Draining lymph nodes were harvested 7 days after immunization was terminated. Lymph nodes were cultured in vitro, then stimulated or unstimulated with cognate peptides for 20 hours, and then analyzed by ELISpot. Spleens were obtained from individual mice two (2) weeks after the last immunization (vaccination), and the CMI of IFN-secreting splenocytes was assessed using an ELISpot assay (Gabitzsch ES, et al. Cancer Immunity Antigens 2010;59: 1131-35 (Cancer Immunol Immunother. 2010; 59:1131-35); Gabitzsch ES, et al. Cancer Gene Ther. 2011; 18:326-35 (Cancer Gene Ther. 2011; 18:326-35); Jones FR , et al. Vaccinology 2011;29:7020-26 (Vaccine 2011;29:7020-26)).
Embodiment 3
[0507] Vaccination with Ad5[E1-,E2b-]-vector containing Alkbh6.2, Slit3 and Atxn10.1 in
[0508] Generation of cell-mediated immune responses against these neo-epitopes in vivo
[0509] Each group of five (5) mice was subcutaneously administered at two (2) week intervals at a dose of 1 x 10 8 , 1×10 9 or 1×10 10 VP Ad5[E1-, E2b-]-Alkbh6.2, Slit3 and Atxn10.1 or blank control vector. One week later, mice were challenged intradermally with 300,000 live CMS5-FFLuc tumor cells. Tumor growth was monitored using live bioluminescent imaging (PerkinElmer). Blood was collected from mice, T cells were isolated, and stimulated in vitro with cognate peptides, unstimulated or unstimulated with unrelated peptides. Media were analyzed for IFN-γ and IL-2 production. Flow cytometry of mouse T cells for intracellular IFN-γ production.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com