Method for preparing ionic liquid and application thereof
An ionic liquid and anion technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
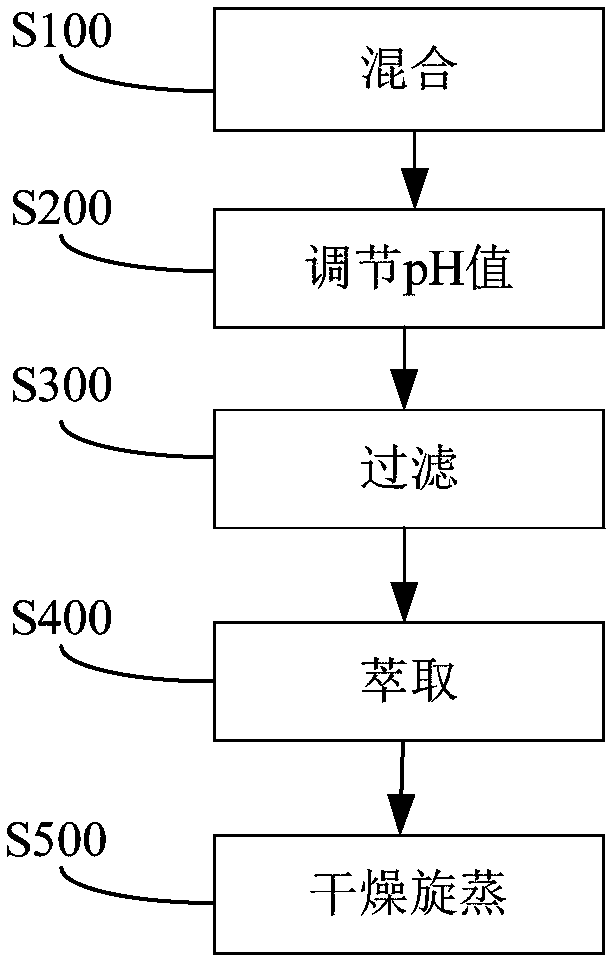
Embodiment 1
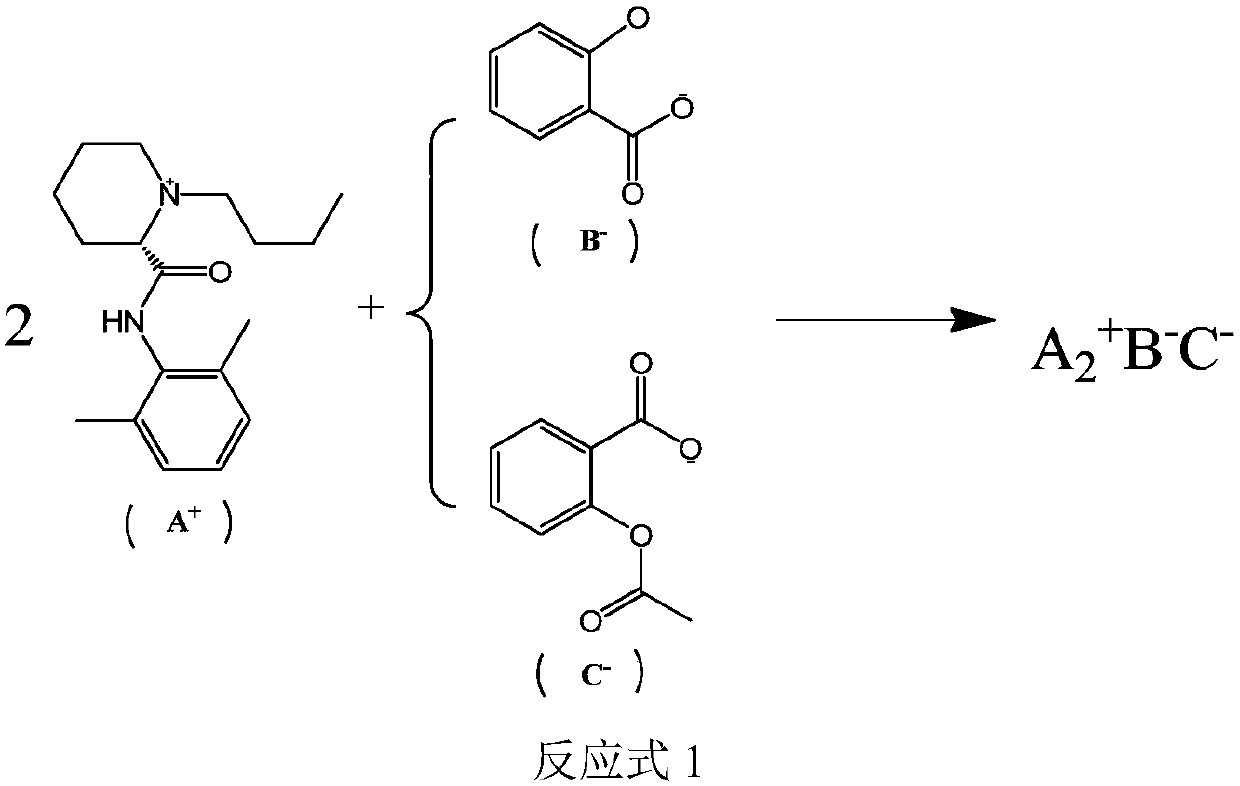
[0058] Utilize the method of the embodiment of the present invention to prepare levobupivacaine-salicylic acid ionic liquid, the specific method is as follows:
[0059] 1. Take 4.87g levobupivacaine hydrochloride, 1.80g aspirin and 0.69g salicylic acid into 500ml ethanol solution and stir to dissolve for 40 minutes.
[0060] 2. Control the reaction temperature at 20°C, slowly add 75ml of Na with a concentration of 2mol / l dropwise at a speed of 0.5s / drop 2 CO 3 solution. After the dropwise addition, the solution was stirred for 15 minutes, and the pH value of the solution was detected to be 7.2, and the solution was colorless and transparent.
[0061] 3. Add 2.25 g of L-tartaric acid to the solution in step 2, add acetone and stir, a small amount of precipitate precipitates out, and filter.
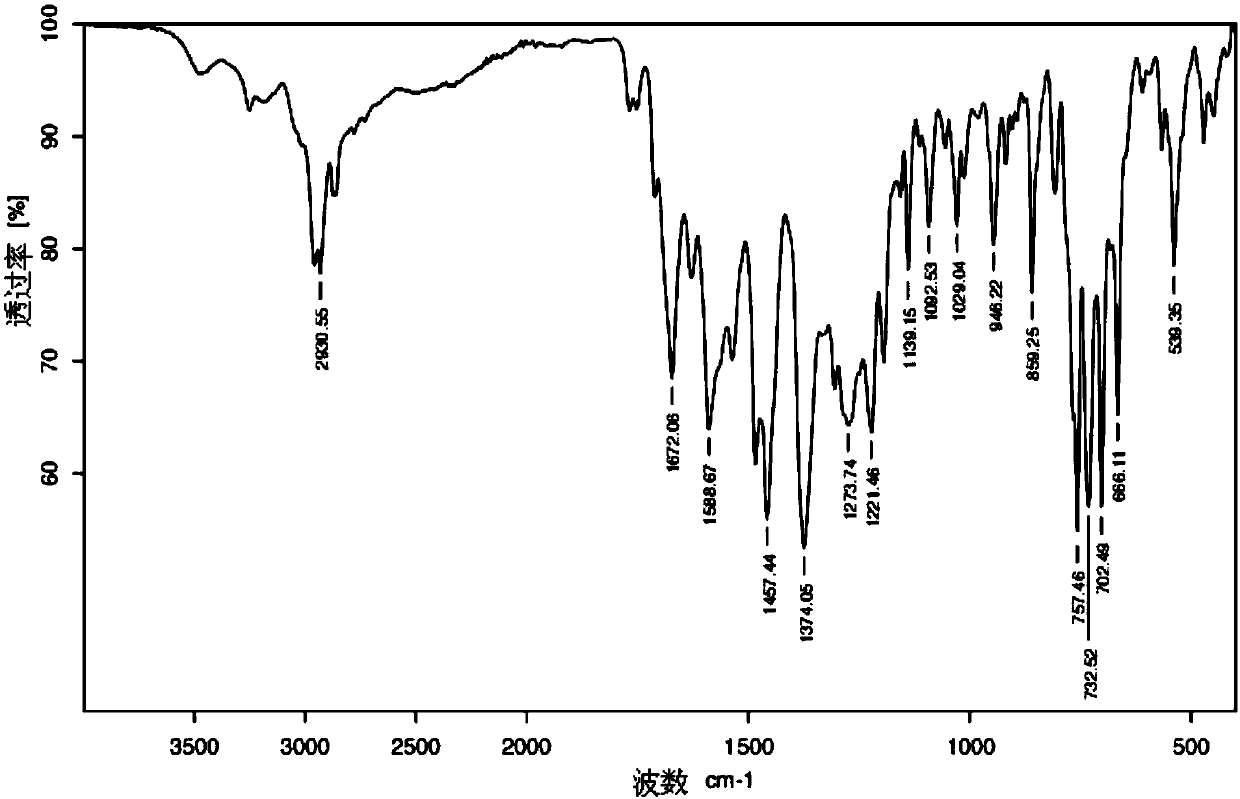
[0062] 4. Add 200ml of ethyl acetate to the filtrate in step 3 for extraction, and rotate the extracted ethyl acetate layer at 30°C under reduced pressure to obtain a colorless, transpa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com