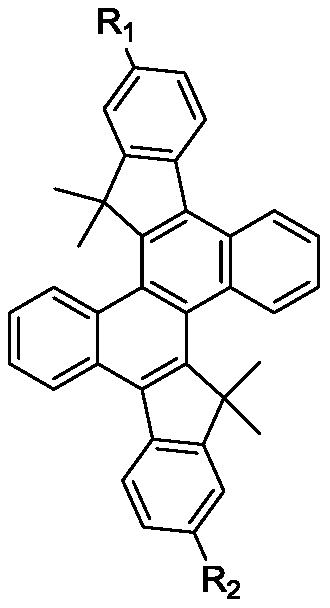
Heterocyclic organic electroluminescent material with chrysene as center structure, and preparation method and application thereof
A central structure, electroluminescence technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of immature blue light-emitting devices, low device life and efficiency, etc., and achieve good film stability, good photoelectricity. performance, the effect of not easy to crystallize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
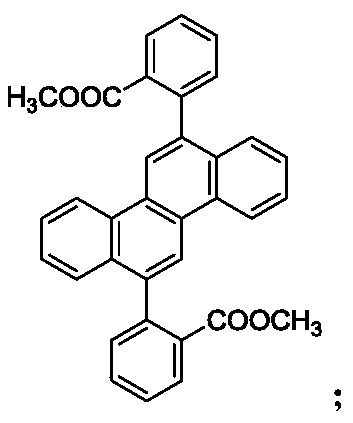
[0050] The preparation of embodiment 1 compound 1
[0051]
[0052] (1) Preparation of Intermediate 1-1: In a 2L three-necked flask, add 6,12-dibromochryl (77.2g, 0.20mol), 2-(methoxycarbonyl)phenylboronic acid (75.6g, 0.42mol) , potassium carbonate (82.8g, 0.60mol), tetrakistriphenylphosphine palladium (4.62g, 4.0mmol) and 800g toluene, 250g water, under the protection of nitrogen, react at 100-110°C for 10h, after the reaction, filter, filter cake Purified by column chromatography, and then recrystallized from toluene to obtain intermediate 1-1 with a yield of 87.64%;
[0053] (2) Preparation of Intermediate 1-2: In a 2L three-necked flask, add Intermediate 1-1 (74.5g, 0.15mol), 600g of tetrahydrofuran dehydrated, and stir to cool down to -50~-60°C under nitrogen protection , add methyllithium ether solution (0.90mol) dropwise, keep the temperature at -50~-60°C for 2 hours after the dropwise addition, then raise the temperature to room temperature and stir for 5 hours. ...
Embodiment 2
[0057] The preparation of embodiment 2 compound C02
[0058]
[0059] In a 250ml three-necked flask, under the protection of nitrogen, add compound 1 (6.18g, 0.01mol), 1-naphthylboronic acid (3.78g, 0.022mol), potassium carbonate (2.76g, 0.03mol), tetrakistriphenylphosphine Palladium (116mg, 0.1mmol), 8mL of water and 80mL of toluene were reacted at 80-90°C for 12h. After the reaction, filtered, purified by filter cake column chromatography, and then recrystallized by toluene and petroleum ether to obtain the target product C02, the yield was 82.19 %.
[0060] High resolution mass spectrum, molecular formula C56H40, theoretical value 712.3130, test value 712.3169.
Embodiment 3
[0061] The preparation of embodiment 3 compound C04
[0062] Referring to Example 2, the raw material 1-naphthaleneboronic acid was replaced with 4-biphenylboronic acid in the preparation process to obtain the target product C04 with a yield of 84.57%.
[0063] High resolution mass spectrum, molecular formula C60H44, theoretical value 764.3443, test value 764.3508.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com