Method for separating and measuring sulfonamide-class impurities in celecoxib through HPLC (High Performance Liquid Chromatography) and application
A technology for sulfonamides and celecoxib, which is used in the separation and determination of sulfonamide impurities in celecoxib by HPLC and the application field, can solve the problems such as the simultaneous separation and quantitative determination of sulfonamides cannot be well achieved, and achieves peak type Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
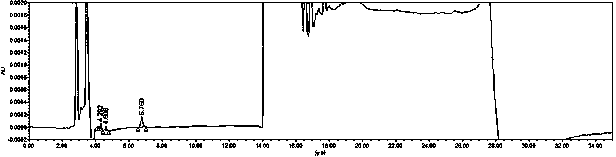
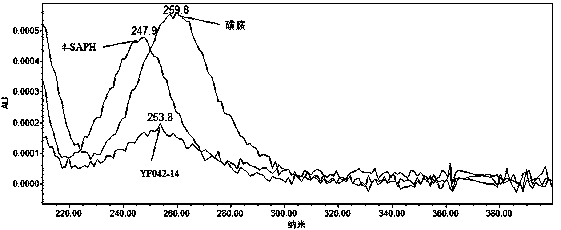
[0061] Embodiment 1: Determination of detection wavelength
[0062] Instrument: Waters e2695-2489 high performance liquid chromatography
[0063] Chromatographic column: phenyl bonded silica gel as filler (Supelcosil LC-DP 4.6*250mm, 5um)
[0064] Mobile phase: Acetonitrile was used as mobile phase A, and 0.05% phosphoric acid solution was used as mobile phase B to carry out gradient elution according to the following table 2:
[0065] time (min)
Mobile phase A (%)
Mobile phase B (%)
0
15
85
15
15
85
20
80
20
25
80
20
30
15
85
35
15
85
[0066] At 0 min, the volume ratio of the inorganic acid in the mobile phase to the organic phase was 85%: 15% (i.e. the initial volume ratio); at 20 min, the volume ratio of the inorganic acid in the mobile phase was reduced to 20%, and the volume ratio of the organic phase The volume ratio increased to 80%; 30 minutes later, the volume ratio of the...
Embodiment 2
[0077] Embodiment 2: the HPLC detection of celecoxib crude drug
[0078] The apparatus and reagents are the same as in Example 1.
[0079] Take about 10 mg each of the sulfonamide reference substance, 4-SAPH reference substance and YF042-14 reference substance, weigh them accurately, place them in 100ml measuring bottles, add diluent to dissolve and dilute to the mark, take 1ml of each of the above three solutions and put them in the same 200ml In the measuring bottle, add diluent to dilute to the mark, and use it as the reference substance stock solution (4-SAPH needs to be newly prepared before use). Precisely pipette 1.5ml of the reference substance stock solution, put it in a 20ml volumetric bottle, add diluent to dilute to the mark, shake well, and use it as the reference substance solution (newly prepared for immediate use).
[0080] Take about 100mg of celecoxib, accurately weighed, put in a 10ml measuring bottle, add 2ml of acetonitrile to dissolve, then dilute to the...
Embodiment 3
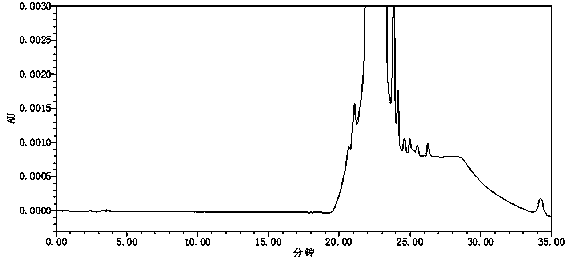
[0091] Embodiment 3: system suitability test
[0092] The apparatus and reagents are the same as in Example 1. The system applicability of this embodiment also inspected the system applicability of the instrument during continuous sampling. Since 4-SAPH is unstable, only the other two impurities, sulfonamide and YF042-14, were used to investigate the applicability of the instrument system.
[0093] Take about 10 mg each of the sulfonamide reference substance and YF042-14 reference substance, weigh them accurately, place them in 100ml measuring bottles, add diluent to dissolve and dilute to the mark, take 1ml of each of the above two solutions and put them in the same 200ml measuring bottle, add diluent diluted to volume as a system suitability stock solution. Precisely pipette 1.5ml of the reference substance stock solution, put it in a 20ml measuring bottle, add diluent to dilute to the mark, shake well, and use it as a system suitability solution.
[0094] Take the system ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com