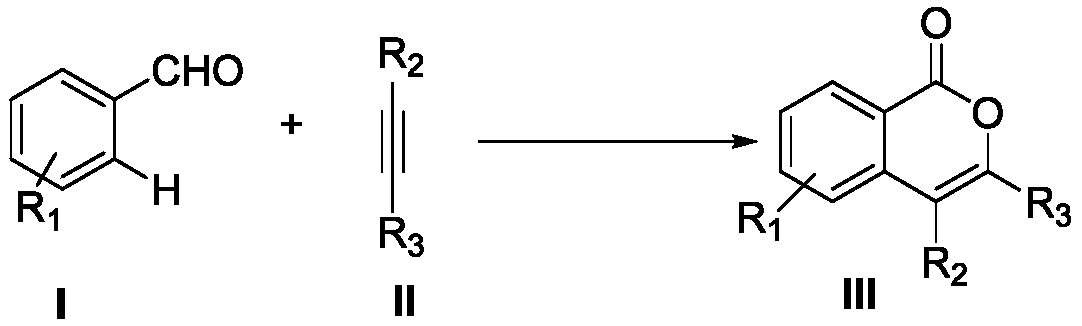
Synthesis method of isocoumarin compounds
A technology of isocoumarin and synthesis method, applied in chemical recovery, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0041] Embodiment 1-15 reaction condition optimization test
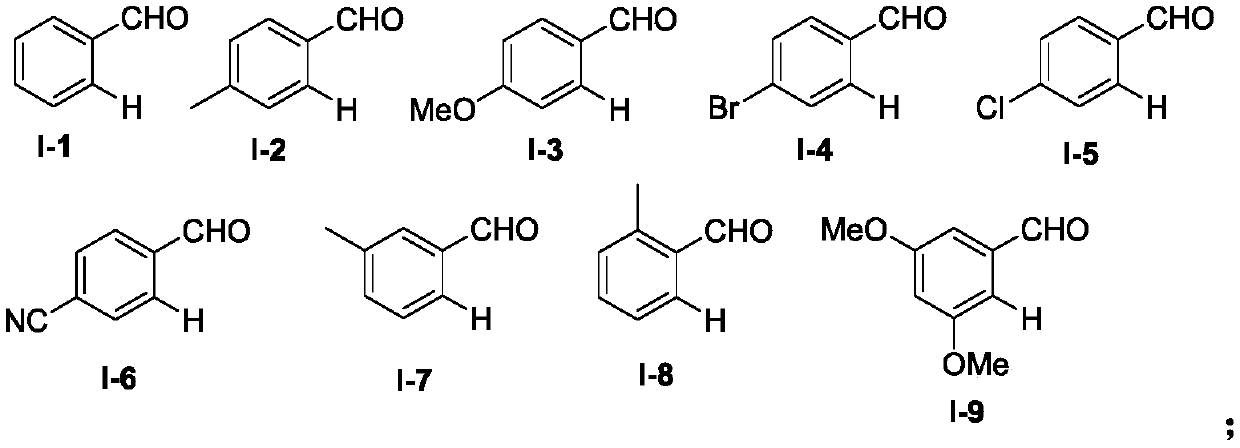
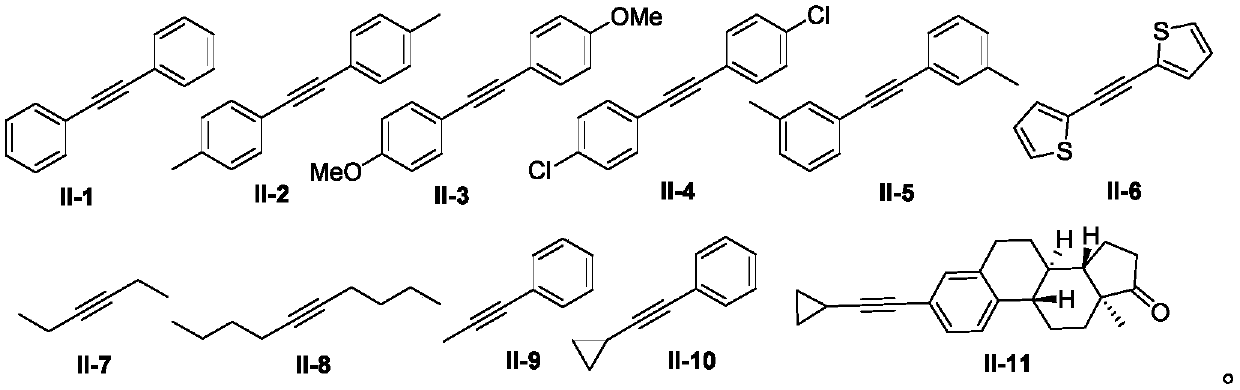
[0042] Using benzaldehyde shown in formula I-1 and diphenylacetylene shown in formula II-1 as templates, the influence of different reaction conditions on the optimization results of the synthesis process was discussed, and representative examples 1-15 were selected. . The results are shown in Table 1.
[0043]
[0044] Wherein the typical test operation of embodiment 1 is as follows:
[0045] In the 25mL Schlenk lock reactor, add benzaldehyde (0.6mmol) shown in formula I-1, diphenylacetylene (0.3mmol) shown in formula II, Cp*Co(CO)I successively 2 (10mol%), copper oxide (2equiv), molecular sieve (100mg), and then add PEG-400 (2g), under oxygen atmosphere (1atm), the oil bath is heated to 100 ℃, after the insulation reaction for 24 hours, by TLC or GC -MS detects that the reaction raw materials are completely consumed. After the reaction was complete, cool to room temperature, extract with diethyl ether (3×10...
Embodiment 34
[0059] Embodiment 34 Catalyst recycling test
[0060] Extracted with ether in Example 1 containing PEG-400, Cp*Co(CO)I 2 , the mixture of CuO and molecular sieves was concentrated in vacuo, added to the Schlenk reactor of 25 mL, and then added successively benzaldehyde (0.6 mmol) shown in formula I-1, diphenylacetylene (0.3 mmol) shown in formula II ), under an oxygen atmosphere (1atm), the oil bath was heated to 100° C., and after 24 hours of insulation reaction, the reaction raw materials were completely consumed by TLC or GC-MS detection. After the reaction was complete, cool to room temperature, extract with diethyl ether (3×10 mL), combine the diethyl ether phases and wash with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate in vacuo to give a residue. Then, the residue was separated by silica gel column chromatography (the elution phase was n-hexane / ethyl acetate) to obtain isocoumarin compounds represented by formula III-1. Yield 82%. The distribution of r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com