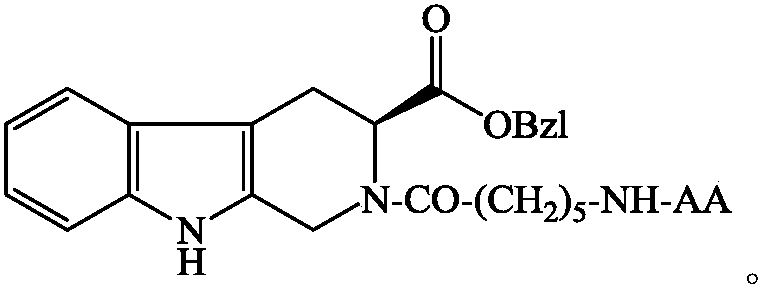
Side chain protected aminoamido n-hexanoyl carboline carboxylic acid benzyl ester, preparation, activity and applications thereof
A technology of aminoamido and tert-butoxycarbonylaminoamido, which is applied in organic chemistry, drug combination, antitumor drugs, etc., and can solve problems such as unsatisfactory antitumor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
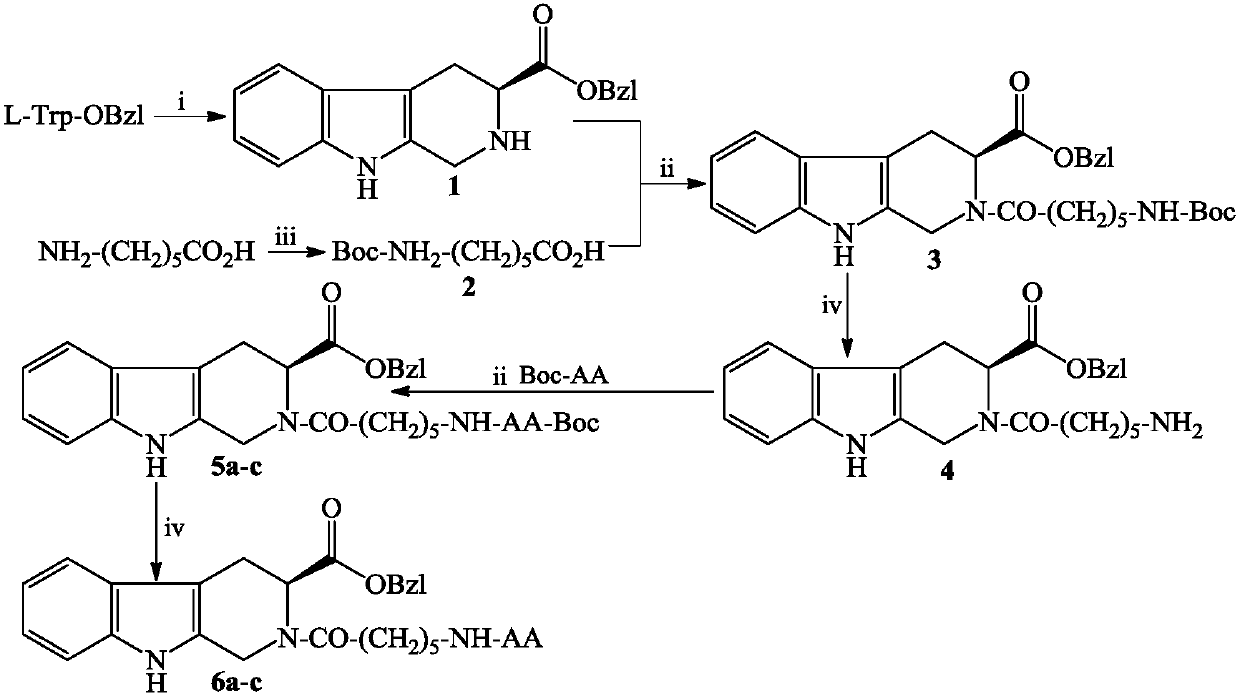
[0017] Example 1 Preparation of (3S)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0018] Slowly add 1.5 mL of concentrated H to 300 mL of water in an ice bath 2 SO 4 , stirred for 3min. Then 5.00 g (15.1 mmol) of L-tryptophan benzyl ester hydrochloride and 6 mL of formaldehyde solution (40%) were added. Stir at room temperature for 72h, TLC showed that L-tryptophan benzyl ester hydrochloride disappeared. The pH of the reaction solution was adjusted to 7 with concentrated ammonia water under an ice bath, and filtered. The filter cake was dissolved with 100 mL of ethyl acetate, and the resulting solution was washed with saturated aqueous sodium chloride solution (40 mL×3), and then dried over anhydrous sodium sulfate. Filter and concentrate the filtrate under reduced pressure. The residue was purified by silica gel column chromatography (dichloromethane:methanol=90:1) to obtain 1.9 g (41%) of the title compound as a yellow oily solid. ESI-MS(m / e): 30...
Embodiment 2
[0019] Embodiment 2 prepares 6-tert-butoxycarbonylaminocaproic acid (2)
[0020] 5.00 g (38.2 mmol) of 6-aminocaproic acid (EACA) were suspended in 50 mL of distilled water. Add 10 mL of aqueous sodium hydroxide solution (2M) to the obtained suspension under ice-cooling, and stir until dissolved. Add 9.2g (42.2 mmol) (Boc) to the resulting solution 2 A solution of O and 20 mL of dioxane was adjusted to pH 9 with aqueous sodium hydroxide solution (2M), and stirred for 30 min. Remove the ice bath and stir at room temperature until TLC shows that 6-aminocaproic acid disappears. The reaction solution was washed with saturated KHSO under ice bath 4 The aqueous solution was adjusted to pH 7, and concentrated under reduced pressure to remove dioxane. The reaction solution was washed with saturated KHSO under ice bath 4 The aqueous solution was adjusted to pH 2 and extracted with ethyl acetate (50 mL×3). The ethyl acetate layer was washed with saturated NaCl aqueous solution (40...
Embodiment 3
[0021] Example 3 Preparation of (3S)-N-(6-tert-butoxycarbonylamino n-hexanoyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (3)
[0022] Dissolve 4.15g (18mmol) tert-butoxycarbonyl-6-aminocaproic acid in 80mL of anhydrous THF, add 2.46g (18.2mmol) N-hydroxybenzotriazole (HOBt) and 4.04g (19.6mmol ) N,N-dicyclohexylcarbodiimide (DCC), stirred for 60 minutes. Afterwards, a solution of 5.06 g (16.5 mmol) (3S)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester and 80 mL of anhydrous tetrahydrofuran was added to the obtained reaction liquid. The reaction mixture was adjusted to pH 9 with N-methylmorpholine (NMM) in an ice bath, and stirred at room temperature for 24 hours. TLC showed the disappearance of benzyl (3S)-2,3,4,9-tetrahydro-β-carboline-3-carboxylate. The reaction solution was filtered, the filtrate was concentrated under reduced pressure, and the residue was dissolved in ethyl acetate. The solution was sequentially washed with saturated NaH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com