Loaded catalyst and method of directly converting synthesized gas to prepare low carbon olefin
一种催化剂、合成气的技术,应用在催化剂活化/制备、催化剂、分子筛催化剂等方向,能够解决产物低碳烯烃选择性低、选择性降低等问题,达到提高转化率、促进活化转化、优异选择性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of Catalyst Component I
[0032] (1) Synthesis of ZnO materials with high specific surface area by precipitation method:
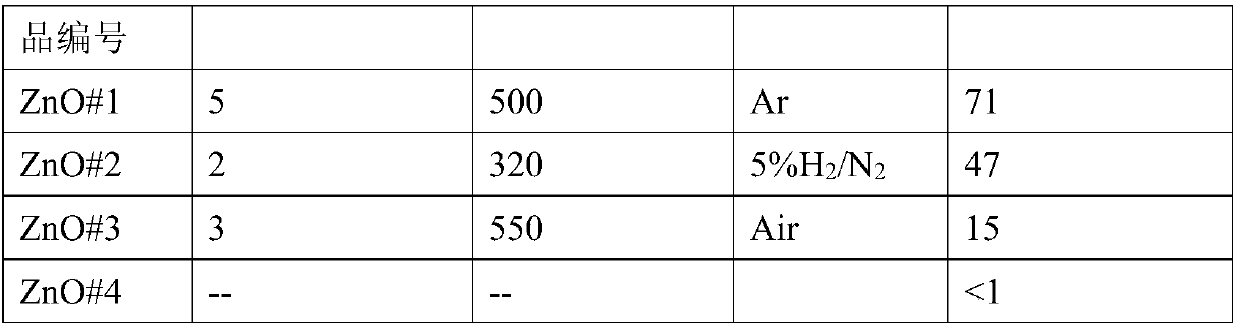
[0033] (1) Weigh 3 parts, 0.446g (1.5mmol) Zn(NO 3 ) 2 ·6H 2 O in 3 containers, then weigh 0.300g (7.5mmol), 0.480g (12mmol), 0.720g (18mmol) NaOH and add them to the above 3 containers in turn, then add 30ml of deionized water to each of the 3 containers In the container, stir at 70°C for more than 0.5h to make the solution evenly mixed, and cool naturally to room temperature. The reaction solution was centrifuged to collect the precipitate after centrifugation, and washed twice with deionized water to obtain the ZnO metal oxide precursor;
[0034] (2) Roasting: After the above-mentioned obtained product is dried in the air, it is calcined in the atmosphere to obtain a ZnO material with a high specific surface area. The atmosphere is inert gas, reducing gas or oxidizing gas; the inert gas is N 2 One or more of , He and Ar; the ...
Embodiment 2
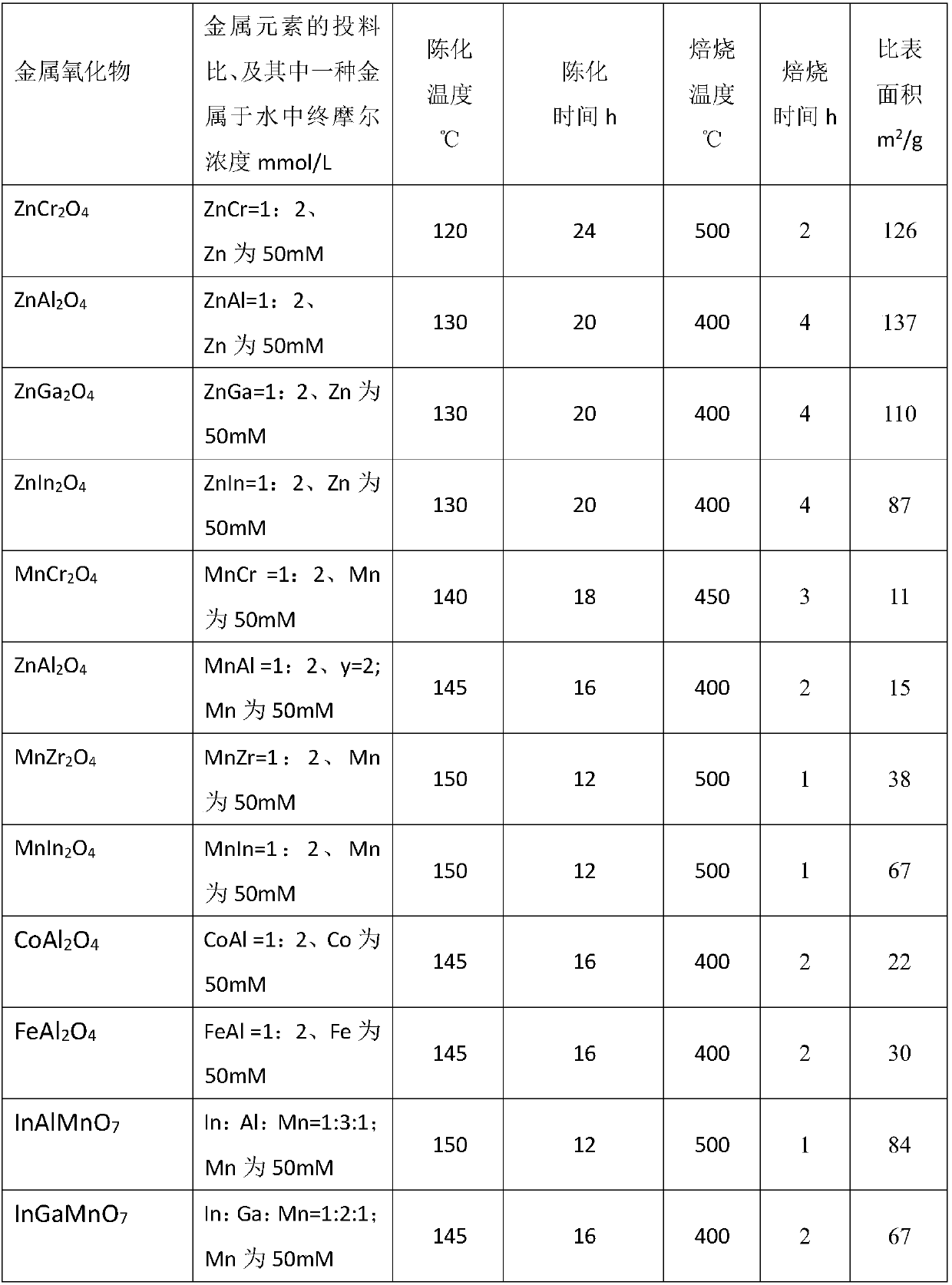
[0108] Examples 2, 6, and 16 have carried out life investigations and found that all three samples can keep the conversion rate, conversion rate and selectivity unchanged in the 700h stability test. Compared with the literature (Jiao et al., Science 351(2016) 1065-1068), the catalyst used in the literature was tested for stability at 650h. Although the conversion rate remained stable, the selectivity of low-carbon olefins changed significantly. , and methane selectivity increased. It shows that the catalyst of the present invention has very obvious advantages in stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com