A class of coumarin hydrazone compound, its preparation method and application
A compound, coumarin technology, applied in chemical instruments and methods, organic chemistry, material excitation analysis, etc., can solve the problems of disturbing endogenous formaldehyde concentration, slow detection response, poor sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
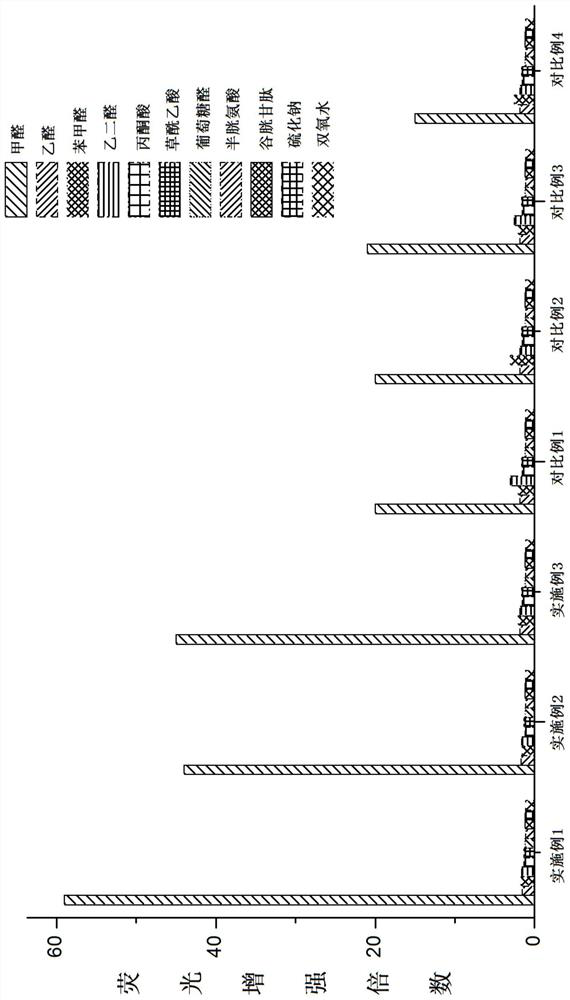
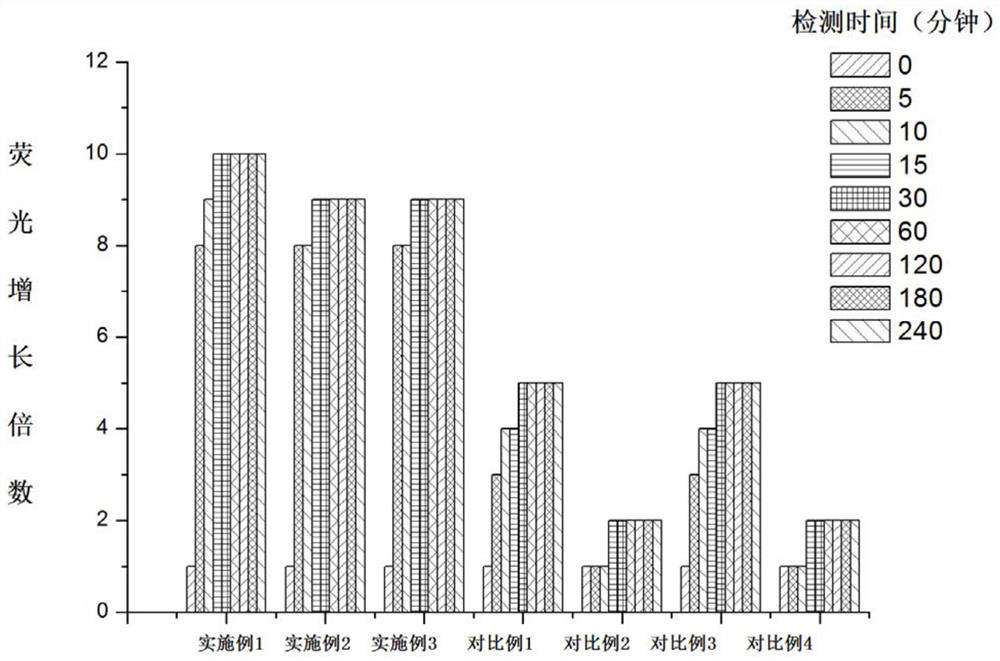
Examples
Embodiment 1
[0055]Example 1: Preparation of I-1
[0056]
[0057]Compound I-1A and Lawson reagents reflow in dried toluene. The reaction ends, cooling filtration, filtration, and dissolved in ethanol after drying solvent, 80% hydrated hydrazine, reflow reaction. After the reaction was completed, the carnivore was added, and the water was added three times with dichloromethal, combined with an organic phase, sodium anhydrous sodium sulfate, and purified, gave I-1 (yield 65%). LC-MS: M / Z 256.145 [M + H]+.
Embodiment 2
[0058]Example 2: Preparation of II-1
[0059]
[0060]Compound II-1A and Lawsen reagents reflow in dry toluene. The reaction ends, cooling filtration, filtration, and dissolved in ethanol after drying solvent, 80% hydrated hydrazine, reflow reaction. After the reaction was completed, the carnifened solvent was added, and the water was added three times with dichloromethydehyde, and the organic phase, sodium alhydrate sodium sulfate was combined, and the column chromatography was purified to give II-1 (yield 68%). LC-MS: M / Z254.1293 [M + H]+.
Embodiment 3
[0061]Example 3: Preparation of II-2
[0062]
[0063]Compound II-2A and Lawn reagents reflow in dry toluene. The reaction ends, cooling filtration, filtration, and dissolved in ethanol after drying solvent, 80% hydrated hydrazine, reflow reaction. After the reaction was completed, the carnifecene was added, and the water was added three times, and the organic phase, dried over anhydrous sodium sulfate was combined, and the column chromatography was purified to give II-2 (yield 73%). LC-MS: M / Z296.1340 [M + H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com