CMKLR1 antagonistic polypeptide and derivative and application thereof
A derivative and antagonistic technology, applied in the field of biotechnology and biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
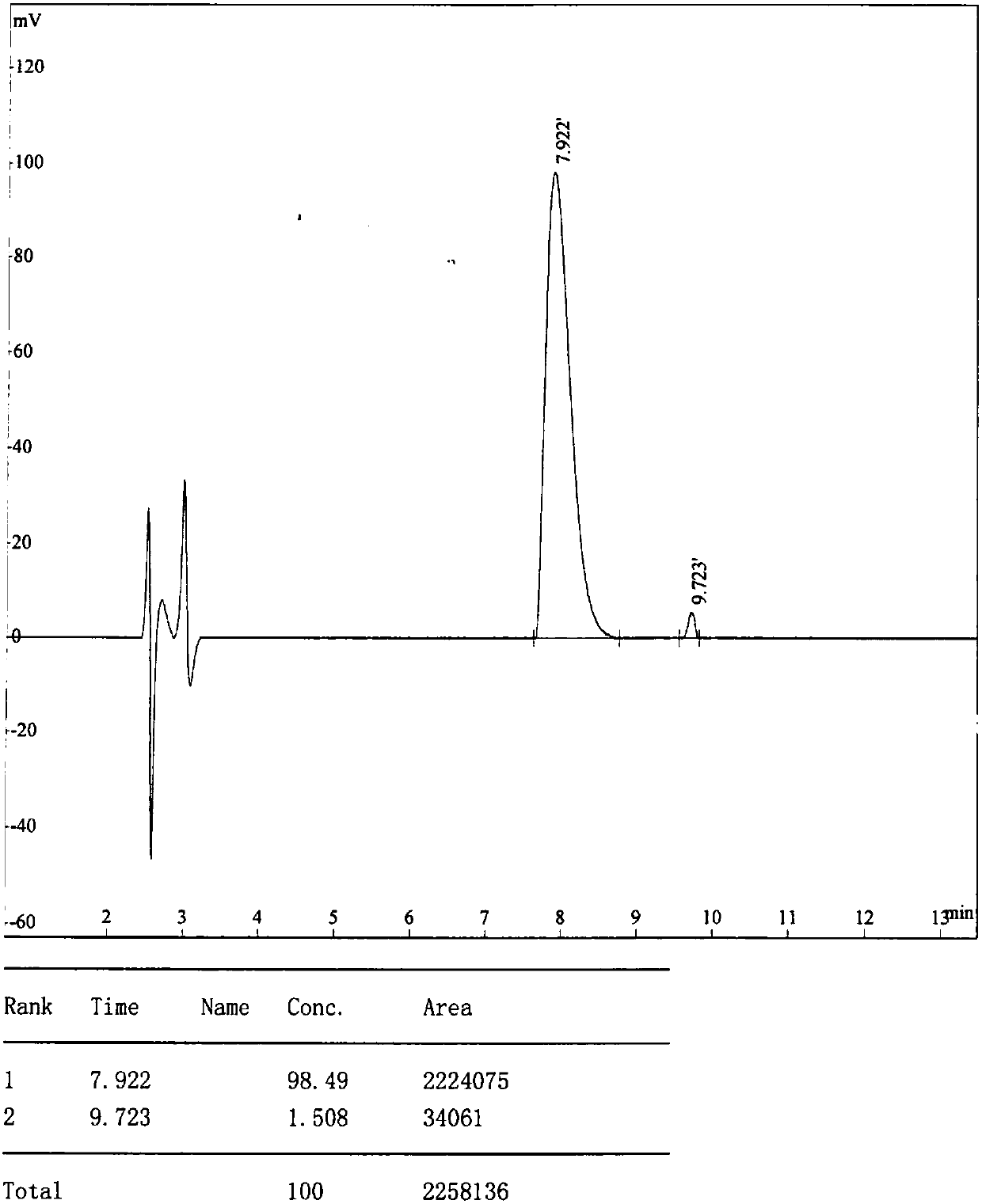
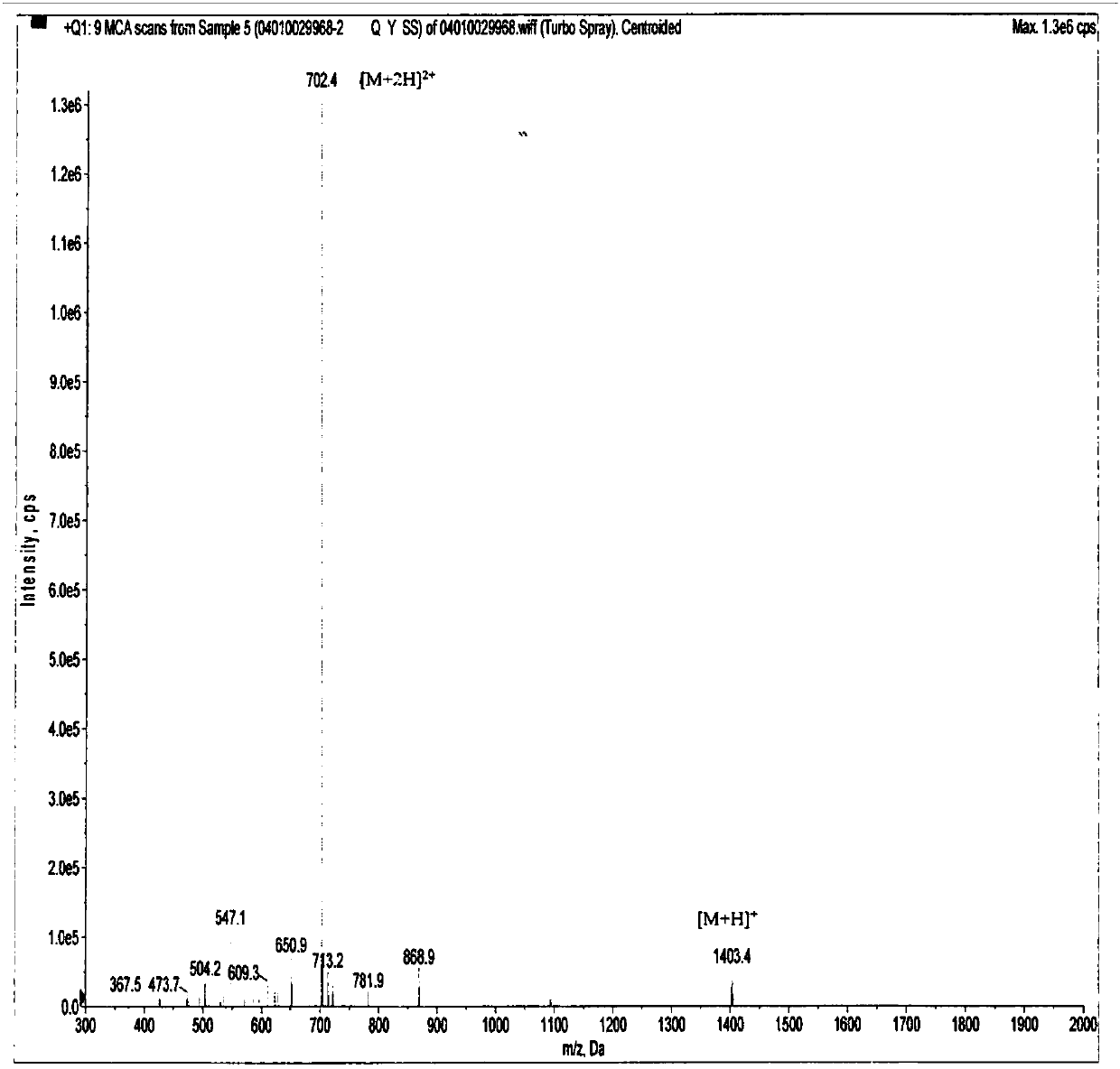
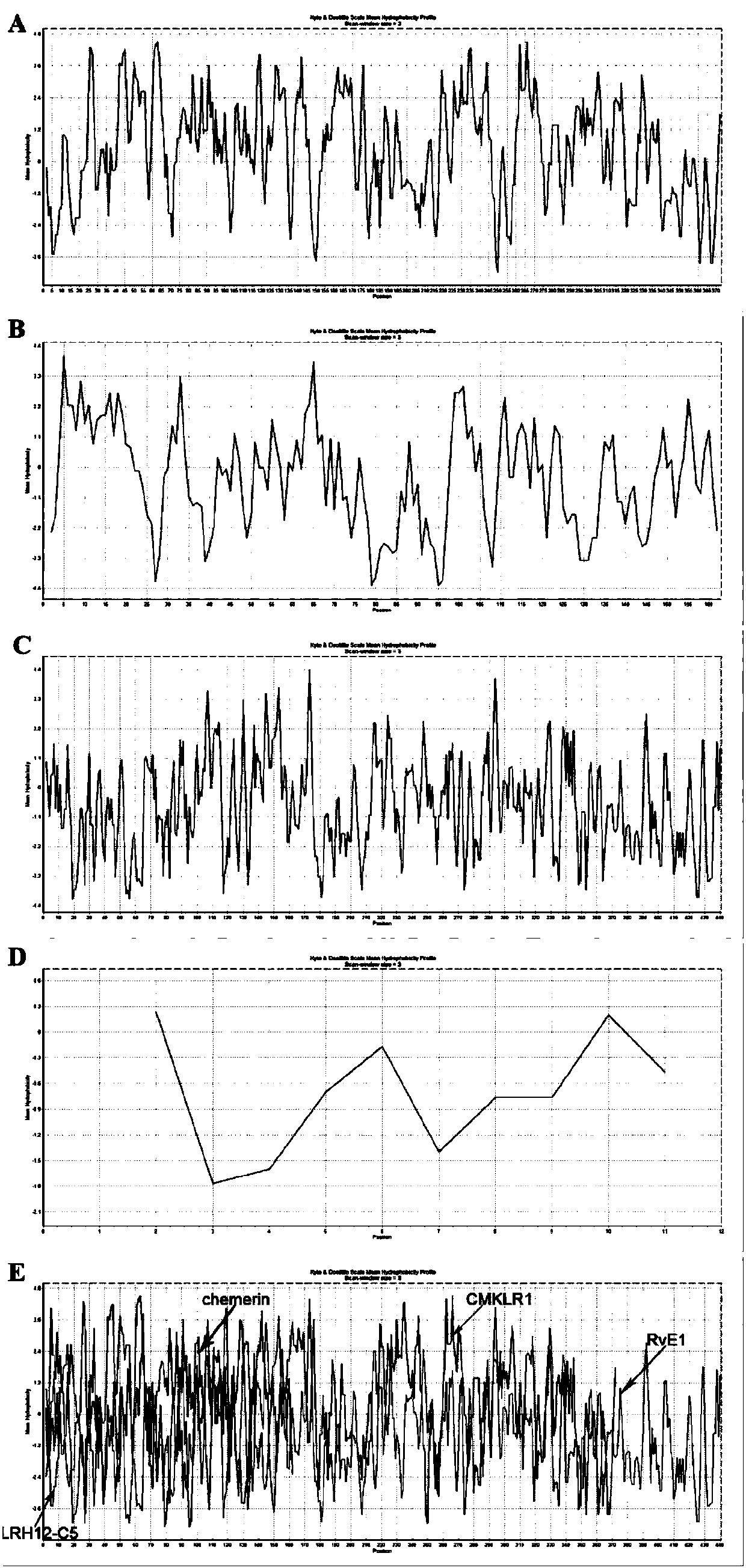
[0172] Example 1: Panning, amplification, purification, sequencing and synthesis of CMKLR1 antagonistic polypeptide LRH12-C5.
[0173] This example is mainly for the purpose of screening positive phages that specifically bind to CMKLR1, and then by amplifying and purifying the positive phages, extracting phage single-stranded DNA (ssDNA) for sequencing, analyzing and comparing the obtained sequences, and finally synthesizing high-purity The antagonistic polypeptide LRH12-C5.
[0174] details as follows:
[0175]1. Establishment of 293T cell line with permanent high expression of CMKLR1: 293T-CMKLR1 + / + / LRH
[0176] ①Select vigorously growing luminescent human 293T cells, and the day before transfection, use 5×10 5 cells / well, inoculated in a 6-well plate, cultured until the second day, the cell fusion degree was 60%;
[0177] ② Transfect on the second day, take one culture well of a 6-well plate as a unit, dilute 3 μg of plasmid with 200 μL of opti-MEM medium, and dilute ...
Embodiment 2
[0194] Example 2 The CMKLR1 antagonistic polypeptide LRH12-C5 can effectively alleviate the inhibitory effect of chemerin on the cAMP signaling pathway.
[0195] (1) Cyclic adenosine monophosphate (cAMP) ELISA:
[0196] ① Cell plating: Wild-type 293T cells and 293T cells highly expressing CMKLR1 (293TCMKLR1 + / + ), with 5x10 5 Each well was inoculated in a 6-well cell culture plate, and the medium volume of each well was 1 mL. After being placed in an incubator for 24 hours, starved overnight, and LRH12-C5 polypeptides with different concentration gradients (3 μM, 0.3 μM, 0.03 μM) were added. , Fosklin (25μM) and chemerin (30nM) for 6h;
[0197] ②Sample preparation: Add 300 μL of cell lysate to each well, place at 4°C for 20 minutes, scrape and collect cells with a cell scraper, mix them upside down, centrifuge at 12,000 rpm for 10 minutes, and collect the supernatant;
[0198] ③ Determination of sample concentration: the sample concentration is determined by BCA method;
...
Embodiment 3
[0206] Example 3 CMKLR1 antagonistic polypeptide LRH12-C5 can effectively inhibit calcium (Ca 2+ ) inflow effect.
[0207] ① Cell plating: Wild-type 293T cells and 293T cells highly expressing CMKLR1 (293T CMKLR1 + / + ), with 5x10 3 Each cell / well was inoculated in a 96-well cell culture plate, the volume of medium in each well was 200 μL, placed in an incubator for 24 hours, and then starved overnight;
[0208] ②Reagent configuration: dissolve probenecid into 1mL buffer solution to prepare probenecid with a concentration of 250nM, shake well, add to fluorescent reagent for use;
[0209] ③Remove the cell culture medium, add LRH12-C5 polypeptide and chemerin (0.3nM) with different concentration gradients (30μM, 3μM, 0.3μM, 0.03μM, 0.003μM) for 30 minutes, and then add 100μL of the above fluorescent reagent to each well;
[0210] ④ Place at 37°C for 30 minutes, then at room temperature for 30 minutes;
[0211] ⑤Measure the fluorescence absorbance at excitation light 494nm and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com