LAMP detection method and kit for mycoplasma hominis
A technology of Mycoplasma hominis and detection kit, which is applied in the biological field, can solve the problems that liquid culture medium cannot accurately judge Mycoplasma hominis infection, cannot achieve pure separation, cannot judge at the same time, etc., and achieves simple, cheap, repeatable, shortened The effect of detection time and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 1. Genomic DNA extraction
[0072] Balance the strains stored at -70°C at room temperature and inoculate them into mycoplasma solid medium and liquid medium, cultivate them in a 37°C incubator for about 36-48 hours, take the bacteria in the liquid medium and wash them with PBS to make 1ml of bacterial suspension liquid. The next steps were performed according to the instructions of the bacterial genomic DNA extraction kit, and the concentration and purity of the extracted samples were measured on a DNA concentration tester.
[0073] 2. LAMP primer design and synthesis
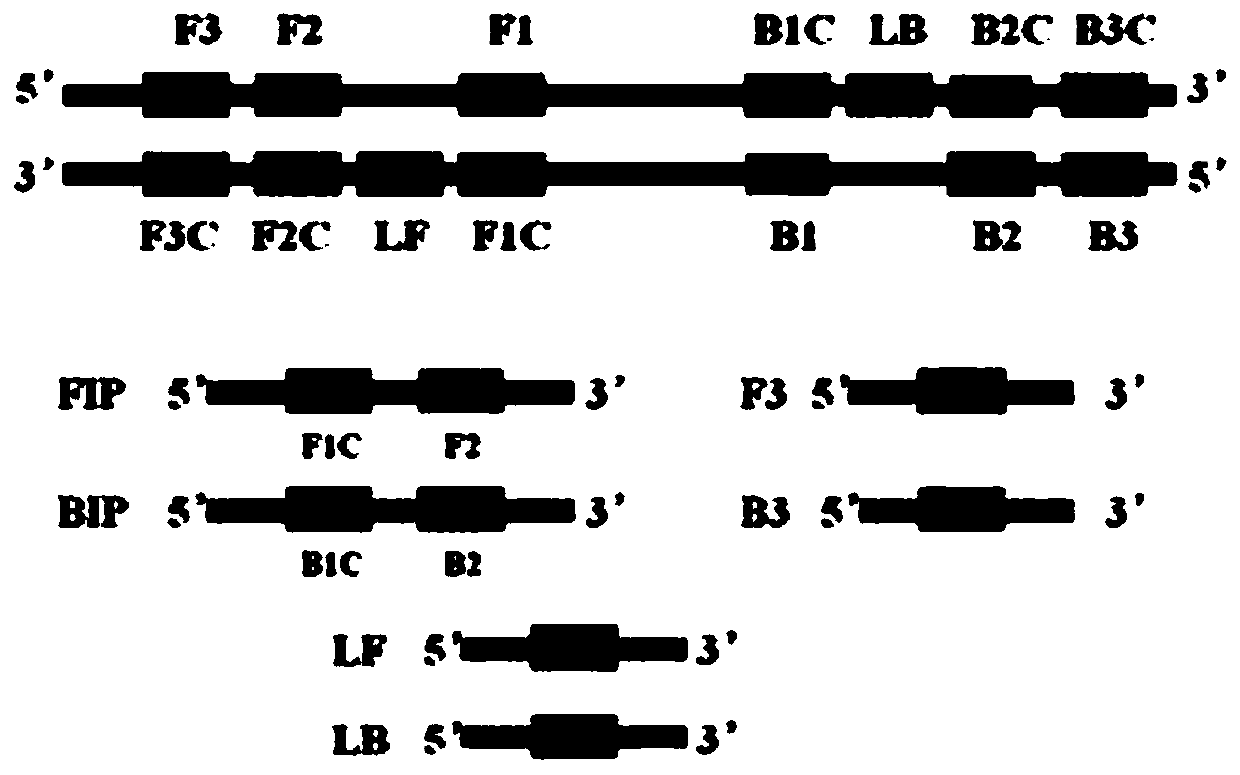
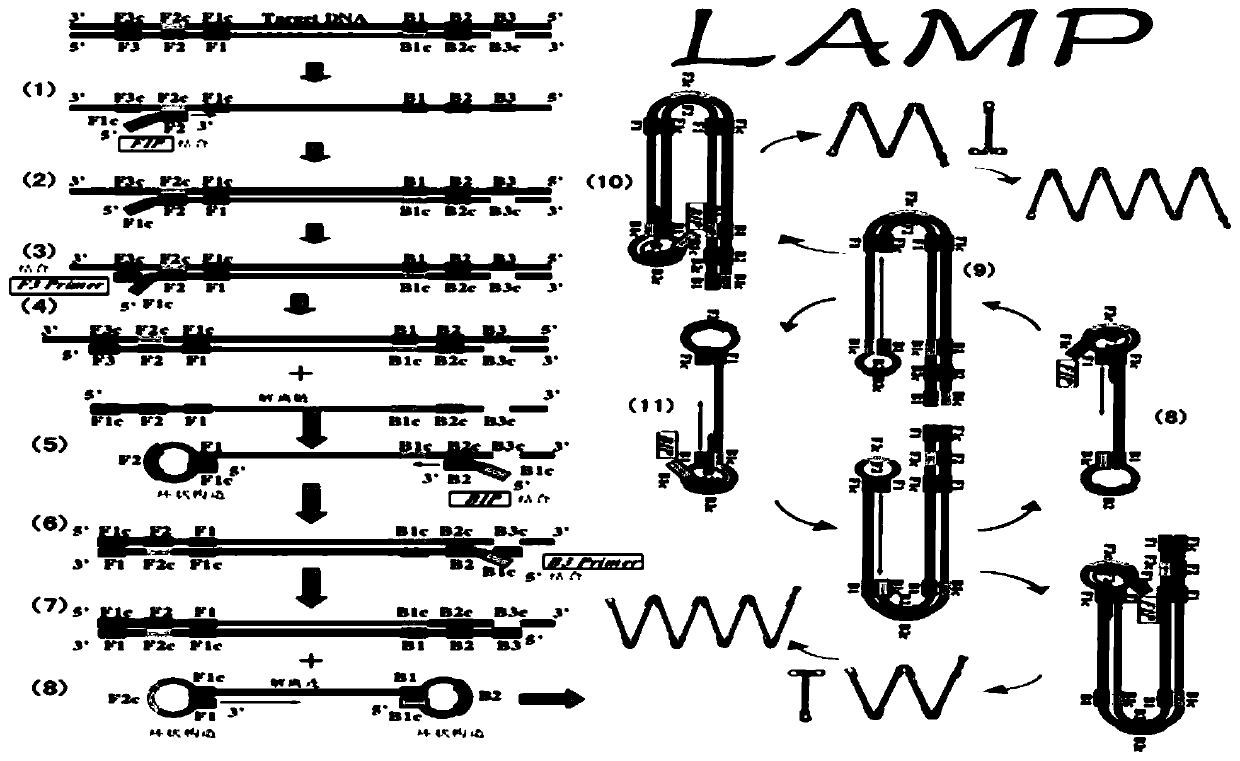
[0074] (1) According to the gap genes gene sequence (SEQ ID NO.1) of Mycoplasma hominis (Mh), primers were designed. Such as figure 1 shown.
[0075] The gap genes gene sequence (SEQ ID NO.25, 444bp) is:
[0076] ATGACAACAGTCCACTCATATACAGCAGACCAAAGATTACAAGATGCTCCACACAAAGATTTAAGAAGAGCAAGAGCAGCTGCTTCAAACATGGTTCCAACAACAACTGGTGCTGCAATAGCAATTGGAAAGGTTATCCCTTCATTGGATAAAAAACTAAATGGACTATCTTTAAGAGTTCCAACAATA...
Embodiment 2
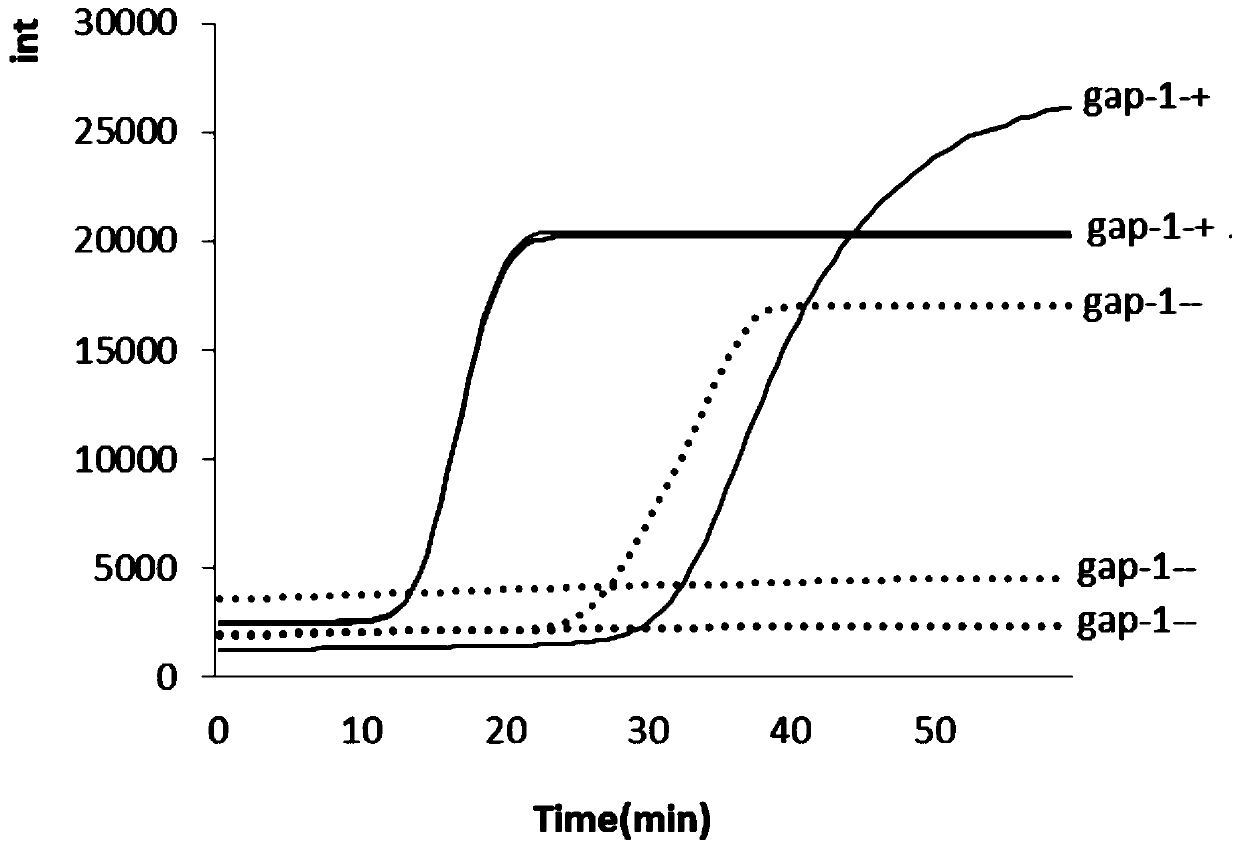
[0099] 1. Specificity test
[0100] Genomic DNA of Mycoplasma hominis and other common clinical pathogens was extracted, amplified according to the reaction conditions of Real-LAMP, the detection signal was analyzed, and the specificity of the primer was evaluated.
[0101] Other relevant clinical bacteria of Mycoplasma hominis are: Staphylococcus hemolyticus, Escherichia coli, Pseudomonas aeruginosa, Mycoplasma hominis, Klebsiella pneumoniae, Proteus mirabilis, Streptococcus agalactiae, Enterococcus aeruginosa. The result is as Figure 9 shown. It can be seen that the common pathogenic bacteria selected in the test of the Mycoplasma hominis system did not appear to be amplified, and the DNA of Mycoplasma hominis could be detected, indicating that the specificity of the primers for the detection of Mycoplasma hominis is good, and it is different from non-target bacteria. There is cross-reactivity.
[0102] 2. Sensitivity test
[0103] To measure the concentration of the DN...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com