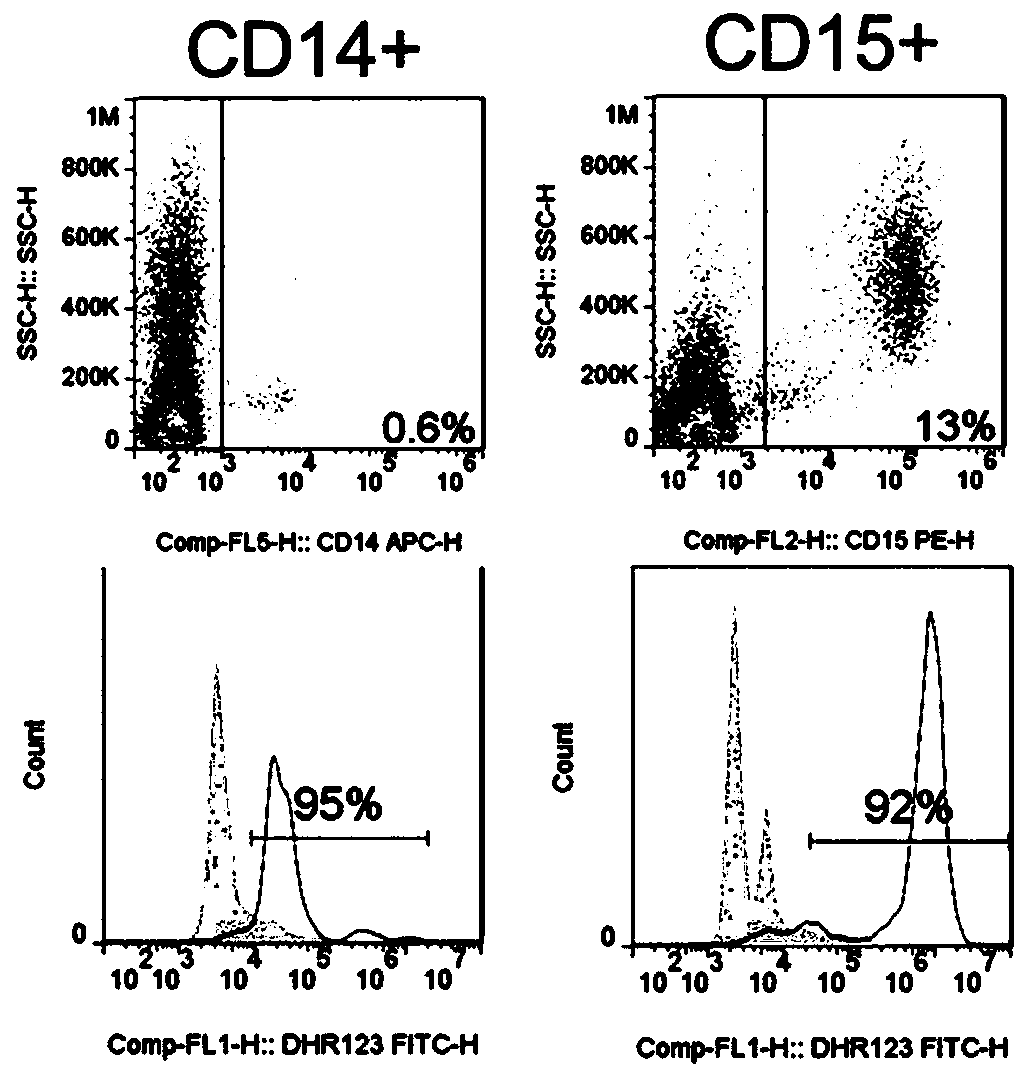
CYBB (cytochrome B-245 beta chain) lentiviral vector, lentiviral vector-transfected stem cells and preparation method and application of lentiviral vector-transfected stem cells
A lentiviral vector and lentiviral technology, applied in the field of genetic engineering, can solve problems such as safety concerns, inability to express for a long time, foreign genes cannot be expressed in appropriate regions, etc., to ensure safety, improve expression efficiency and expression amount , the effect of increasing the amount of expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
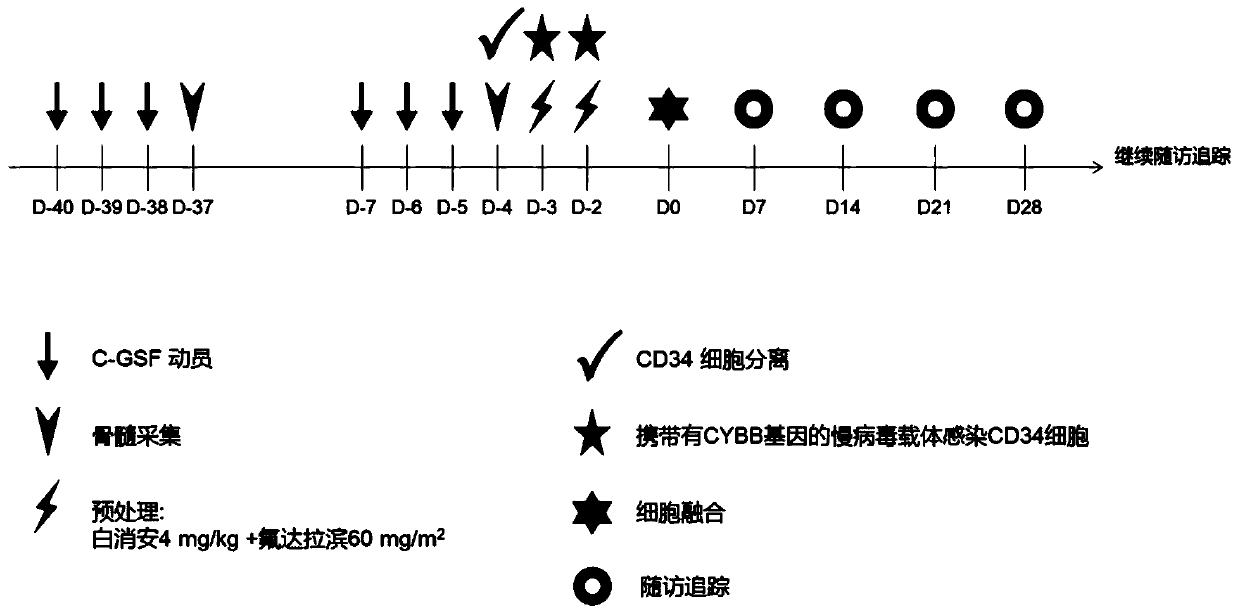
[0073] Example 1 Construction of the lentiviral vector carrying the CYBB gene
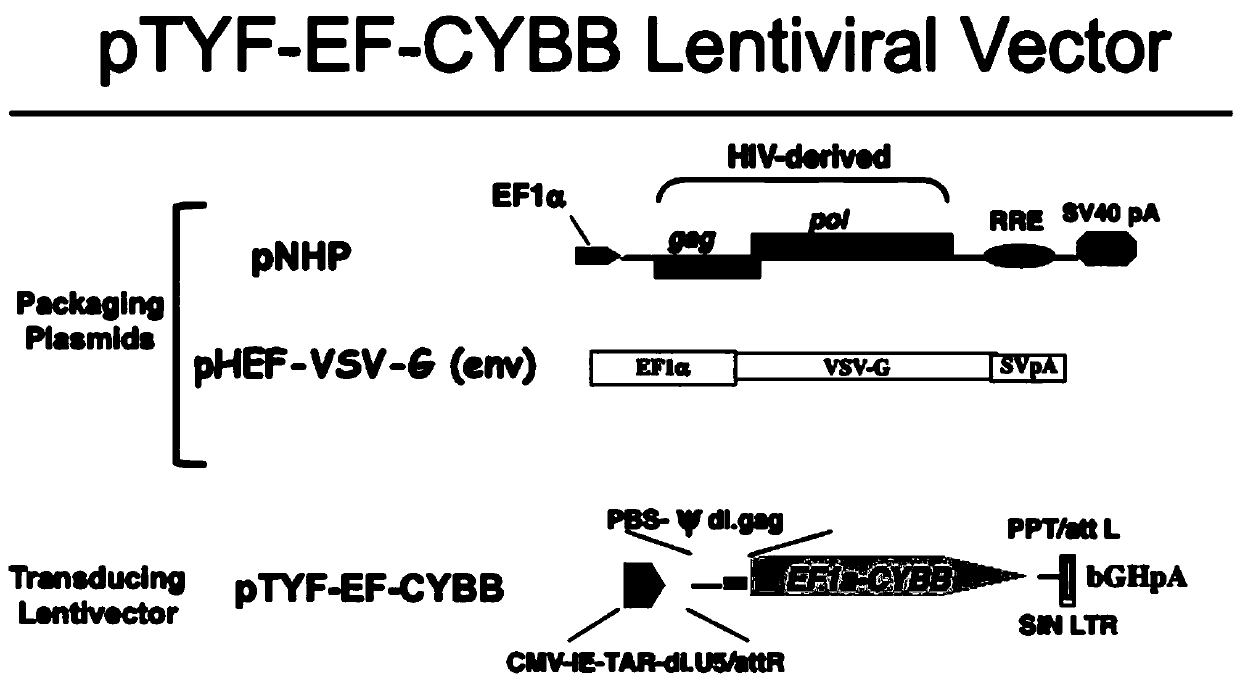
[0074] Synthesize the normal CYBB gene sequence (amino acid sequence as shown in SEQ ID NO.2, nucleic acid sequence as shown in SEQ ID NO.3) through the whole gene synthesis into the TYF-EF1α lentiviral vector (NHP / TYF lentivirusvector system), located behind the human EF1α (hEF1α) promoter sequence (the nucleic acid sequence is shown in SEQ ID NO.1), by sequencing and double enzyme digestion (5' cloned at the BamHI site, 3' cloned at the SpeI site , the optimal reaction conditions refer to the original NEB factory recommendations) and other methods to identify the obtained products, and obtain the correctly connected lentiviral vector carrying the CYBB gene under the activation of hEF1α. like figure 1 Shown is the NHP / TYF lentiviral vector system, including viral packaging plasmids (NHP, EF-VSV-G) and vector plasmids (pTYF-EF-CYBB), where the packaging plasmids include pNHP and pHEF-VSV-G(env) ,...
Embodiment 2
[0075] Example 2 lentiviral packaging
[0076] In this example, a multi-plasmid packaging system was used to package the lentiviral vector carrying the CYBB gene into a complete lentivirus through 293T cells. The specific steps are:
[0077] (1) Cultivate the 293T cell line for 17-18 hours, add fresh DMEM containing 10% FBS;
[0078] (2) Add DMEM, pNHP, pHEF-VSV-G and the lentiviral vector constructed in Example 1 in sequence in a sterile centrifuge tube, and vortex;
[0079] (3) Add Superfect Transfection Reagent (QIAGEN) into the centrifuge tube and let stand at room temperature for 7-10min;
[0080] (4) Add the lentiviral vector-Superfect mixture in the centrifuge tube dropwise to the 293T cells, vortex well, and store at 37°C, 5% CO 2 Cultivate for 4-5 hours;
[0081] (5) Remove the cell culture medium, wash the cells, and add the culture medium to continue culturing;
[0082] (6) Return the culture medium to 5% CO 2 After culturing overnight in an incubator, the tra...
Embodiment 3
[0083] Example 3 Purification and Concentration of Lentivirus
[0084] The purification and concentration process of lentivirus, specifically:
[0085] (1) Lentivirus purification
[0086] The packaged lentivirus was centrifuged at 1000g for 5min to remove cell debris, and the obtained supernatant was filtered with a 0.45μm low protein binding filter, and stored at -80°C after aliquoting;
[0087] (2) Lentivirus concentration
[0088] Add the lentivirus supernatant to the Centricon filter tube and centrifuge at 2500g for 30min; shake the filter tube and centrifuge at 400g for 2min to collect the concentrated virus into a collection cup.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com