Method for directly preparing battery-grade lithium hydroxide from lithium phosphate
A lithium hydroxide, battery-level technology, applied in the direction of lithium oxide;/hydroxide, etc., can solve the problems of low lithium conversion rate and low yield, and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
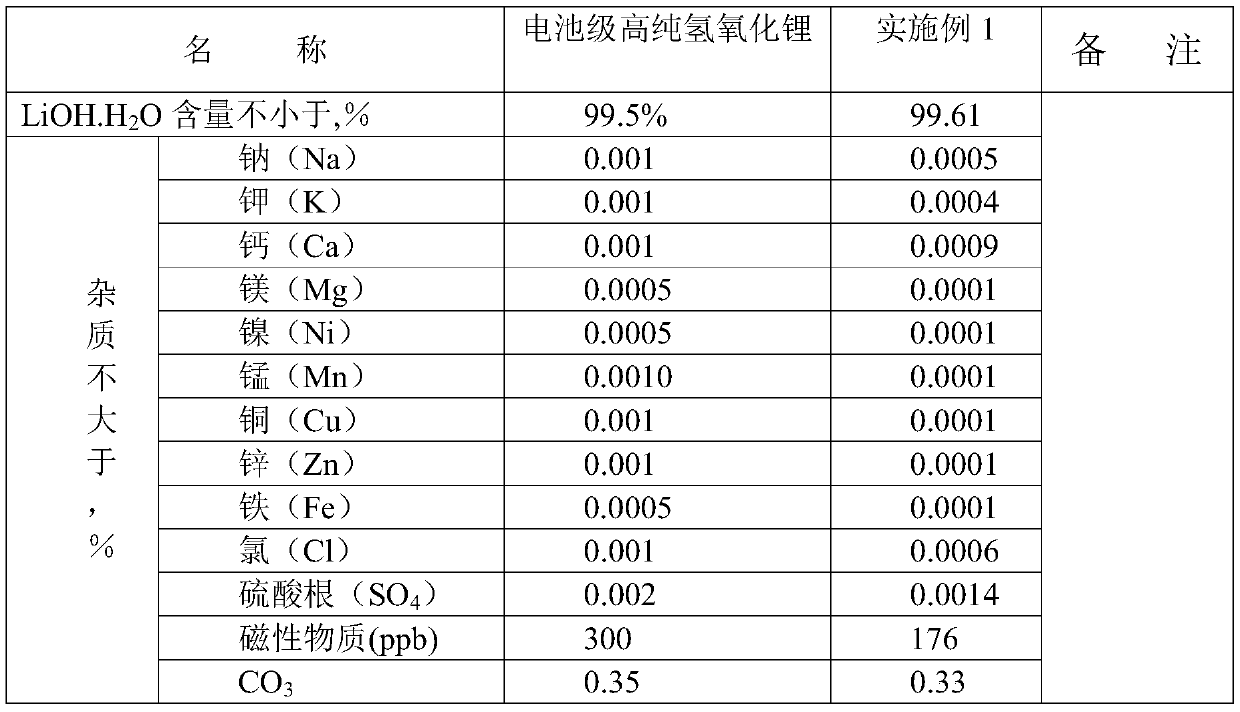
Embodiment 1
[0021] Lithium phosphate used in this example is a dry material, and the content of main impurities such as sodium, potassium, calcium, magnesium, and sulfate radicals is 1-20ppm; sulfuric acid is 98%; barium hydroxide content is 99%.
[0022] (1) Weigh 1000g of lithium phosphate wet material into a 2L beaker, add 1L of pure water, then add 740g of 98% concentrated sulfuric acid until the lithium phosphate wet material is completely dissolved, then add a theoretical amount of 120% of lithium oxalate to precipitate Calcium ions, filtered for later use;
[0023] (2), the filtered solution was evaporated to 155°C in a beaker, and when cooled to 30°C, a large amount of lithium sulfate crystals were precipitated; 1810g of solids were obtained after suction filtration;
[0024] (3), put the lithium sulfate solid of 1810g in poly 3L beaker, add again 1800g pure water, the pH of solution is 3-4, add industrial grade lithium hydroxide 12g to adjust the pH of solution to 7.5, there is ...
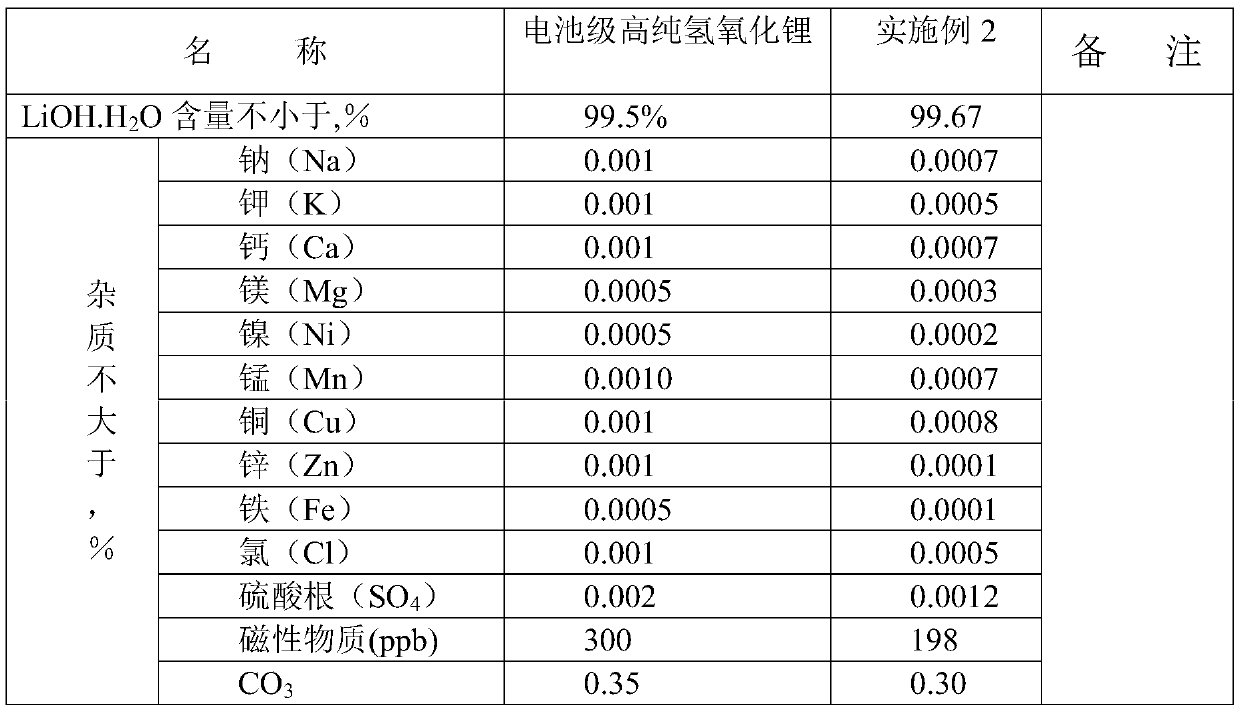
Embodiment 2
[0036] The lithium phosphate wet material content used in this example is 85%, and the content of main impurity such as sodium, potassium, calcium, magnesium, sulfate radical is 1-20ppm; Sulfuric acid is 98%; Barium hydroxide content is 99%.
[0037] (1) Weigh 5000g of lithium phosphate wet material into a 5L PTFE beaker, add 4L of pure water, then add 3550g of 98% concentrated sulfuric acid until the lithium phosphate wet material is completely dissolved, then add a theoretical amount of 120% oxalic acid Lithium precipitated calcium ions, filtered for later use;
[0038] (2), the filtered solution is evaporated to 155°C in a beaker, and when cooled to 30°C, a large amount of lithium sulfate crystals are separated out, and 5220g of solid is obtained after separation with a small centrifuge;
[0039] (3), put the lithium sulfate solid of 5220g in poly 2L beaker, add again 5000g pure water, the pH of solution is 3-4, add industrial grade lithium hydroxide 32g to adjust the pH of...
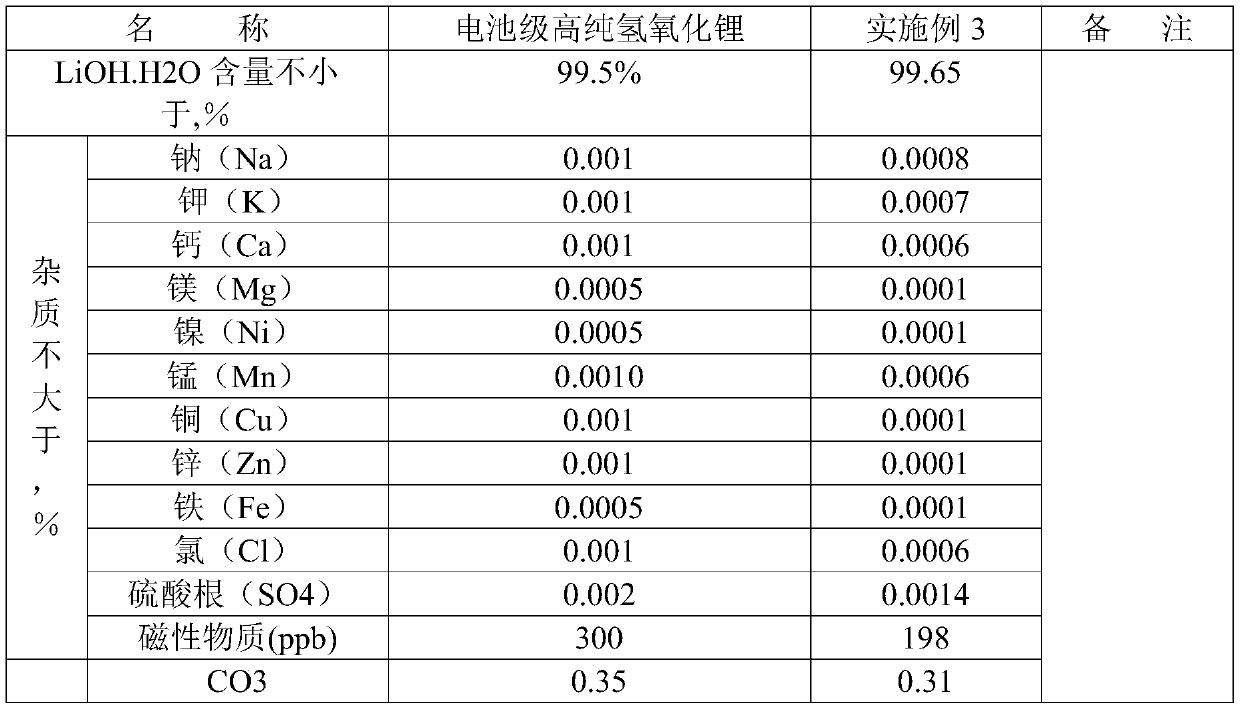
Embodiment 3
[0051] The raw material that this example adopts is identical with example 1, but enlarges input amount
[0052] The lithium phosphate wet material content used in this example is 85%, and the content of main impurity such as sodium, potassium, calcium, magnesium is 1-20ppm; Sulfuric acid is 98%; Barium hydroxide content is 99%.
[0053] (1), weigh 2000kg of lithium phosphate wet material in 3M 3 Add 3000L of pure water and 1620kg of 98% concentrated sulfuric acid until the lithium phosphate is completely dissolved, then add a theoretical amount of 120% of lithium oxalate to precipitate calcium ions, filter until clarified and set aside;
[0054] (2), the filtered solution is evaporated to 155°C in a beaker, and when cooled to 30°C, a large amount of lithium sulfate crystals are separated out; 2713kg of solid is obtained after separation with a centrifuge;
[0055] (3), put 2713kg of lithium sulfate wet material in 3M 3 Add 2000kg of pure water to the enamel reaction kettle an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com