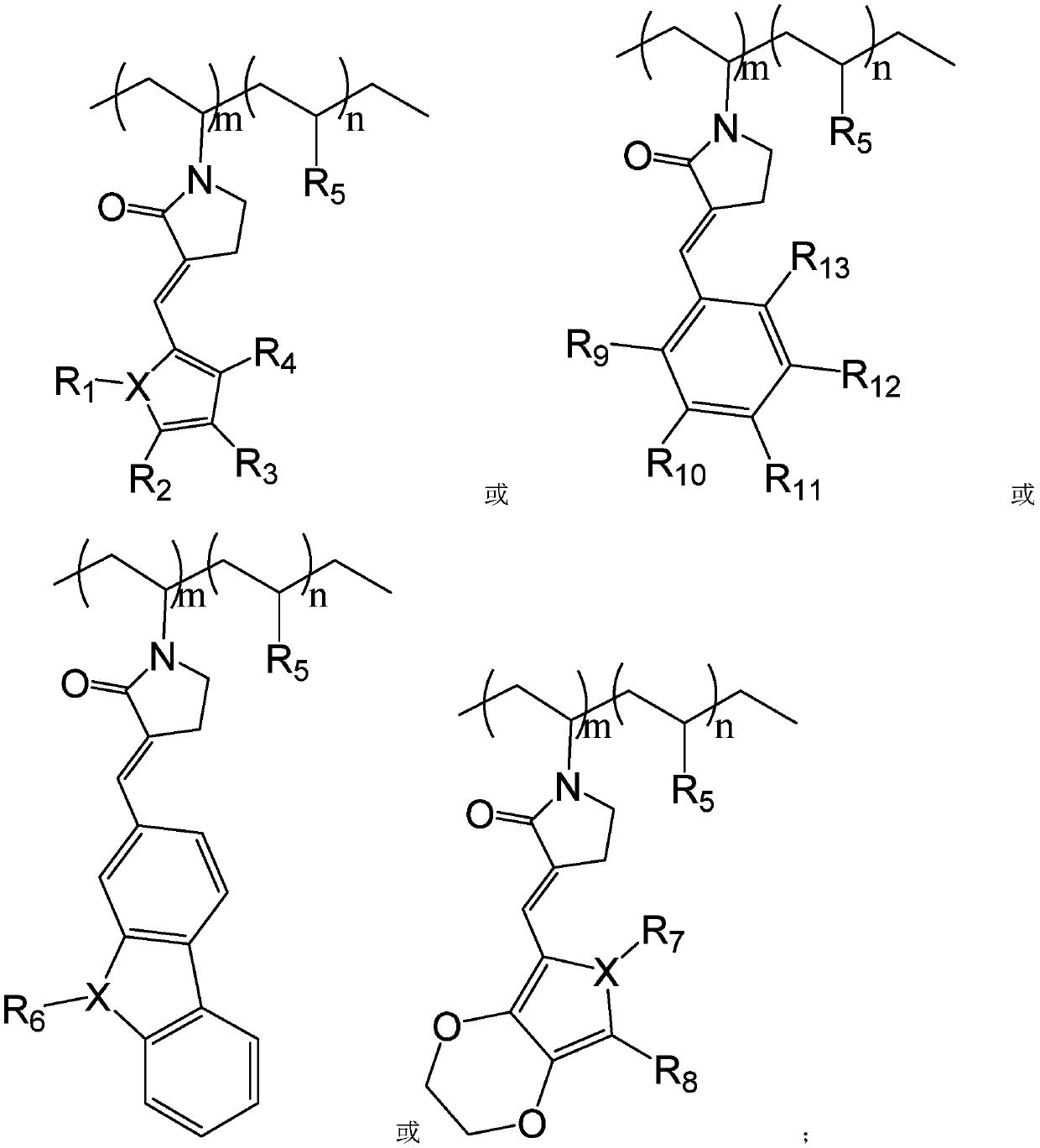
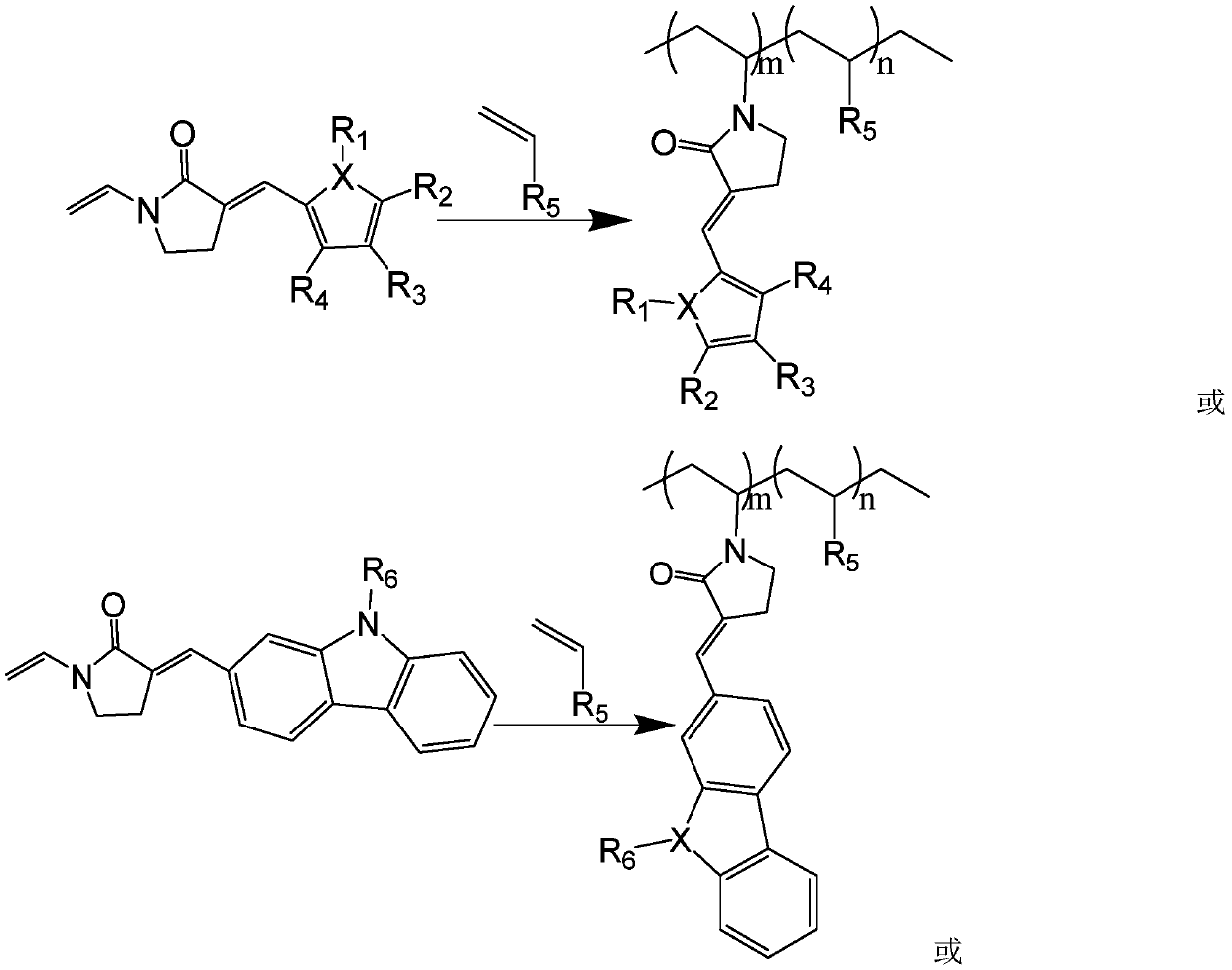
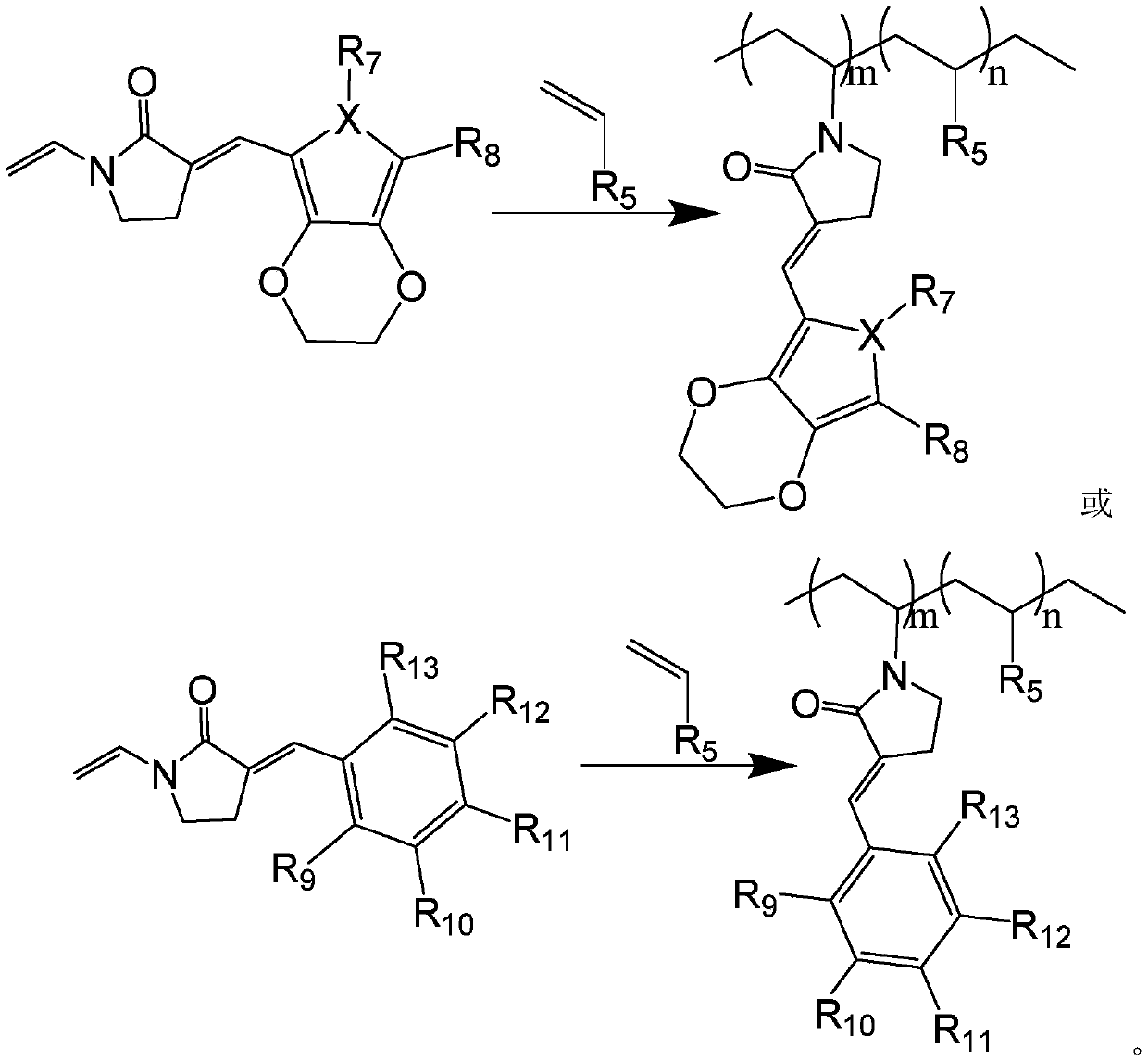
Copolymerized macromolecular photoinitiator and preparation method thereof
A technology of photoinitiator and macromolecule, which is applied in the field of photoinitiator synthesis, can solve the problems affecting the performance of water-based photocuring system, and achieve the effect of improving the problem of oxygen inhibition and low migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A preparation method of a copolymerized macromolecular photoinitiator, comprising the following steps:
[0037] (1) Weigh 1mol furfuraldehyde and 1mol N-vinylpyrrolidone and dissolve them in 20mL of ethyl acetate, and mix them uniformly under stirring;
[0038] (2) Add 3-5 drops of 5% NaOH aqueous solution (0.5gNaOH, 9.5g water) to the mixed solution prepared in step (1), adjust the pH value to 13, react for 3h at 30°C, pass Enter nitrogen, use ice bath to continue reaction 3h, separate out light yellow crystal;
[0039] (3) The light yellow crystals of step (2) are washed with dichloromethane, dried in vacuo to remove the solvent, and the pure light yellow product 2-furan vinyl-N-vinylpyrrolidone is obtained, and its structural formula is
[0040] (4) Dissolve 1mol 2-furyl vinyl-N-vinylpyrrolidone and 0.5mol 1-allylpiperazine in ethanol, add 3-5 drops of saturated sodium hydroxide solution dropwise, blow nitrogen, and heat in a water bath to 50 ℃, reflux, and magne...
Embodiment 2
[0045] A preparation method of a copolymerized macromolecular photoinitiator, comprising the following steps:
[0046] (1) Weigh 1mol of N-ethylcarbazole-3-carbaldehyde and 1mol of N-vinylpyrrolidone, dissolve them in 20mL of ethyl acetate, and mix evenly under stirring;
[0047] (2) Add 3-5 drops of 5% NaOH aqueous solution (0.5gNaOH, 9.5g water) to the mixed solution prepared in step (1), adjust the pH value to 13, react for 3h at 30°C, pass Enter nitrogen, use ice bath to continue reaction 3h, separate out light yellow crystal;
[0048] (3) Wash the light yellow crystals in step (2) with dichloromethane, dry in vacuo to remove the solvent, and obtain a pure light yellow product, which is 2-ethylcarbazolevinyl-N-vinylpyrrolidone, whose structural formula is
[0049] (4) Dissolve the product 2-ethylcarbazolevinyl-N-vinylpyrrolidone obtained in step (3) in methanol, add 3-5 drops of saturated sodium hydroxide solution dropwise, nitrogen, and heat in a water bath to 50 ℃, r...
Embodiment 3
[0054] A preparation method of a copolymerized macromolecular photoinitiator, comprising the following steps:
[0055] (1) Weigh 1mol 3,4-ethylenedioxythiophene-2-carbaldehyde and 1mol N-vinylpyrrolidone, dissolve them in 20mL of ethyl acetate, and mix them uniformly under stirring;
[0056](2) Add 3-5 drops of 5% NaOH aqueous solution (0.5gNaOH, 9.5g water) to the mixed solution prepared in step (1), adjust the pH value to 13, react for 3h at 30°C, pass Enter nitrogen, use ice bath to continue reaction 3h, separate out light yellow crystal;
[0057] (3) Wash the light yellow crystals in step (2) with dichloromethane, dry in vacuo to remove the solvent, and obtain a pure light yellow product, which is 2-(3,4-ethylenedioxythiophene) vinyl-(N-ethylene Base) pyrrolidone, its structural formula is
[0058] (4) Dissolve the product 2-(3,4-ethylenedioxythiophene) vinyl-(N-vinyl)pyrrolidone and 1mol4-vinyl triphenylphosphineamine in methanol, drop Add 3-5 drops of saturated sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com