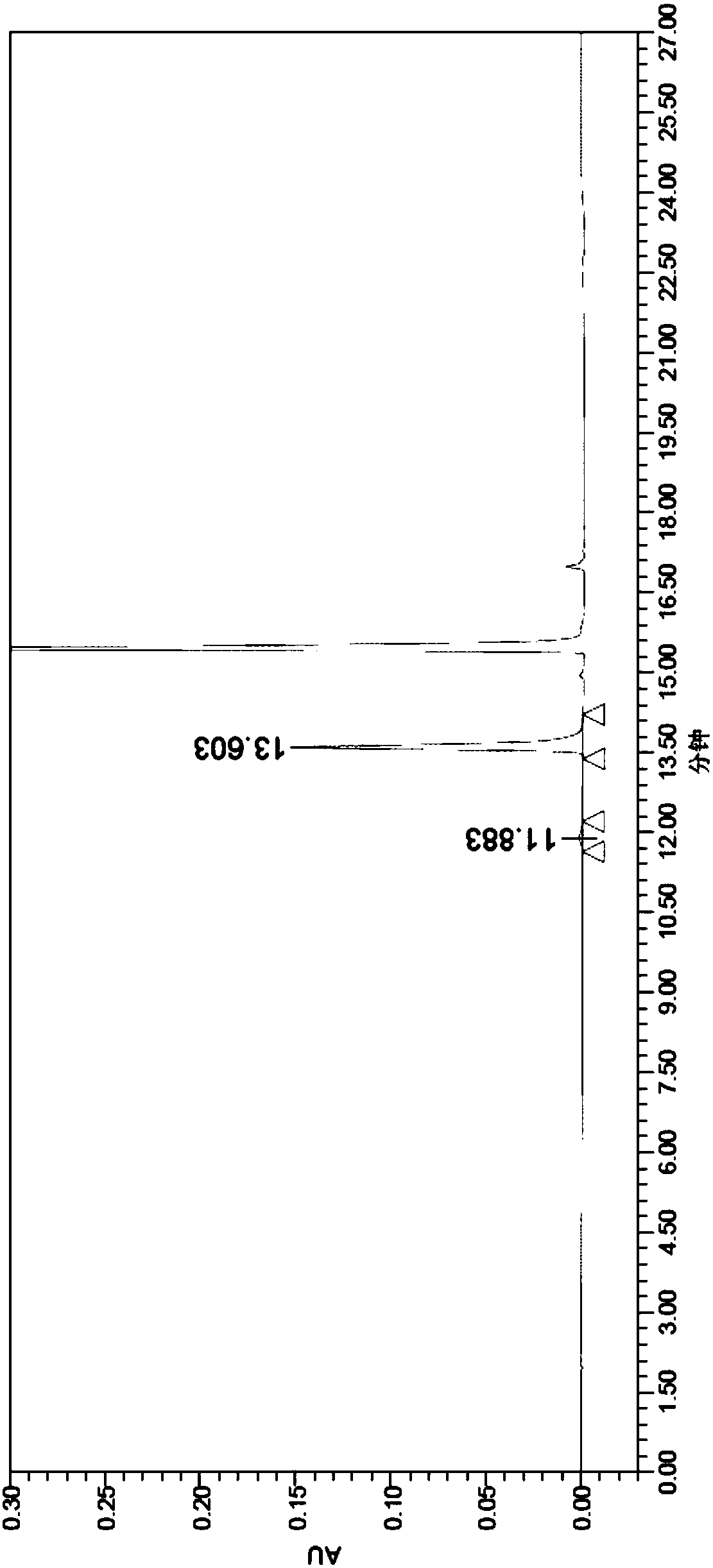
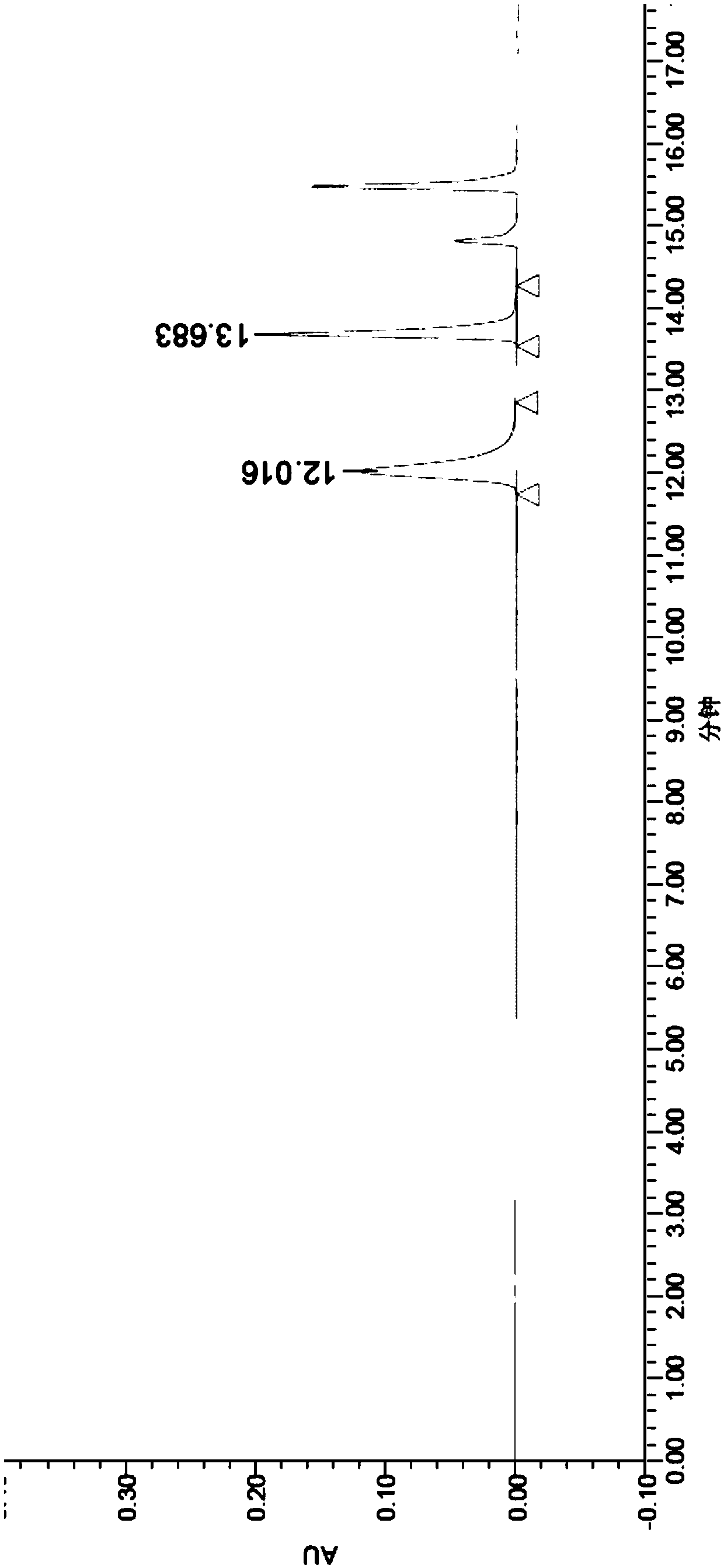
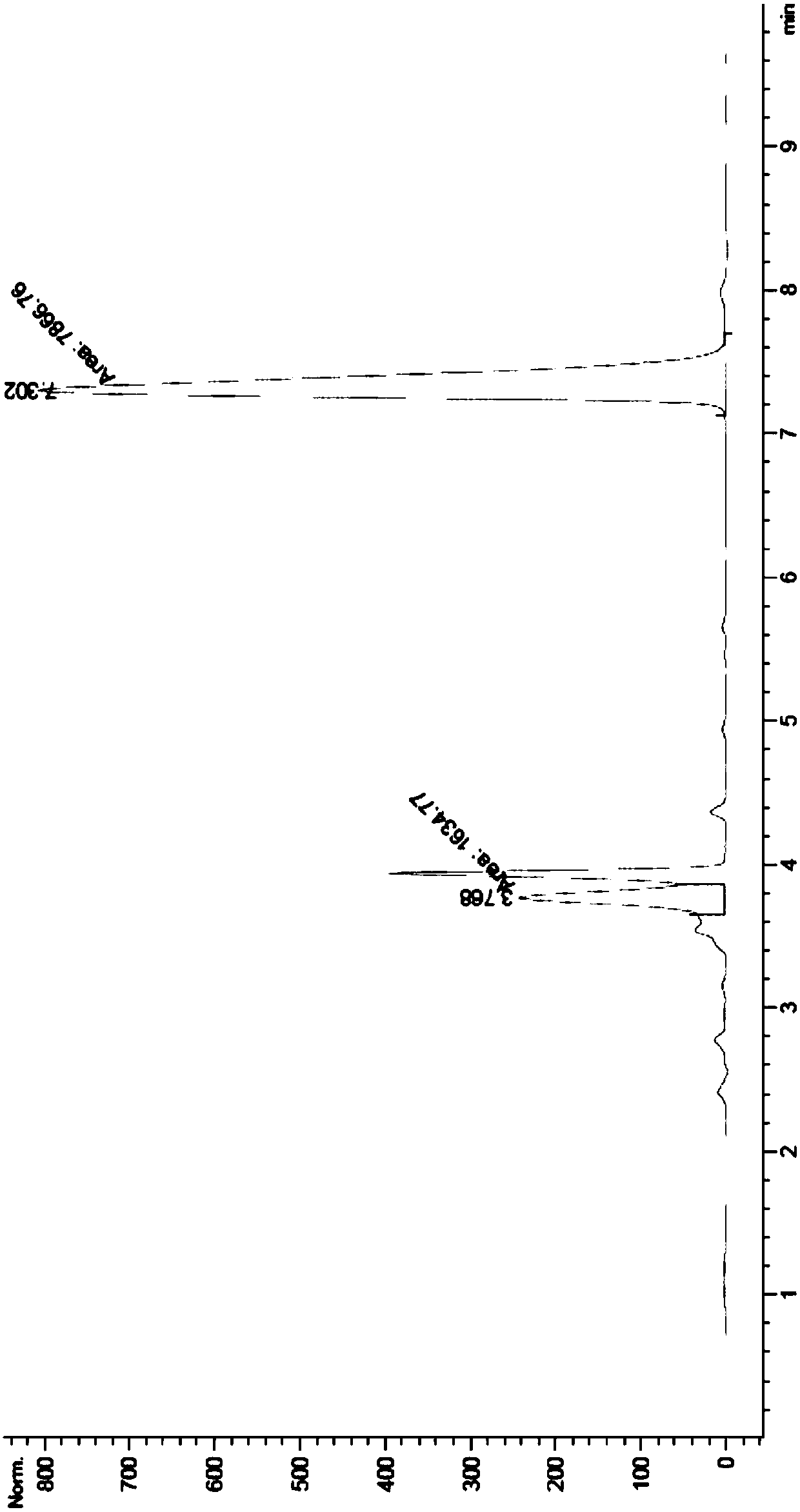
Racemization method of N-acetyl-glufosinate
A technology of glufosinate-ammonium and acetyl, which is applied in the field of racemization of N-acetyl-glufosinate-ammonium salt, can solve the problems of low conversion rate, unfavorable environmental protection, high raw material cost, etc., achieve mild and environmentally friendly reaction conditions, save steps, reduce workload effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Acquisition of N-acetylamino acid racemase gene
[0067] The N-acetylamino acid racemase from Amycolatopsis sp.Ts-1-60 and Amycolatopsisorientalis subsp.lurida microorganisms was retrieved from the NCBI database, and the NCBI accession numbers were 4A6G or CAC00653.1, respectively, and two N-acetylamino acids were synthesized from the whole gene racemase gene.
[0068] Table 1 N-acetyl amino acid racemase
[0069] serial number Types of source NCBI accession number Enz.7 AAR-AT Amycolatopsis sp.Ts-1-60 4A6G Enz.8 AAR-AO Amycolatopsis orientalis subsp. lurida CAC00653.1
Embodiment 2
[0070] Example 2 Expression of N-acetylamino acid racemase gene
[0071] The N-acetyl amino acid racemase gene enzyme is linked to pET28a, the restriction site NdeI&HindIII, and the enzyme-linked vector is transformed into the host Escherichia coli BL21 competent cell. The constructed strains were inoculated into TB culture based on 37°C, 200rpm shaker, IPTG concentration 0.1mM for overnight induction, and the bacteria were harvested.
Embodiment 3
[0072] Example 3 The cultivation of N-acetylamino acid racemase thalline and the preparation of crude enzyme solution and the determination of enzyme activity
[0073] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0074] After the engineering bacteria containing the N-acetylamino acid racemase gene were activated by streaking on the plate, a single colony was picked and inoculated into 5ml LB liquid medium containing 50μg / ml kanamycin, and cultured with shaking at 37°C for 12h. Transfer to 150ml fresh LB liquid medium containing 50μg / ml kanamycin according to 2% inoculum amount, shake at 37°C until OD600 reaches about 0.8, add IPTG to its final concentration of 0.5mM, induce culture at 18°C 16h. After the cultivation, the culture solution was centrifuged at 10,000 rpm for 10 min, the supernatant was discarded, and the bacterial cells were col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com