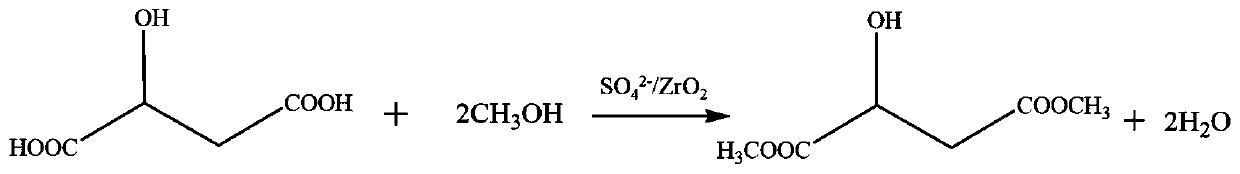Solid superacid catalyst formalic acid esterification
A technology of solid super acid and malate, applied in physical/chemical process catalyst, carboxylate preparation, organic compound preparation, etc., can solve the difficulty of separation between catalyst and reaction system, the difficulty of catalyst recycling, and the pollution of reaction tail liquid. environment and other problems, to achieve the effect of easy recycling, good recycling and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
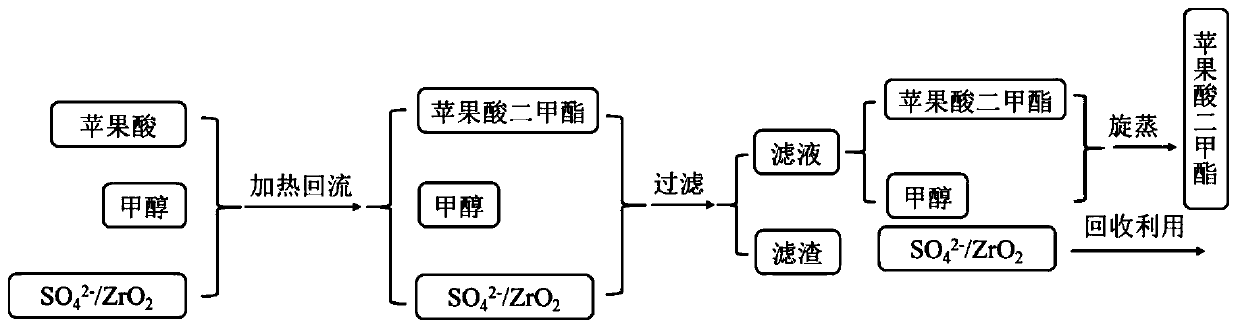
[0024] Solid super acid catalyst SO 4 2- / ZrO 2 Preparation: Put 1g ZrO on the watch glass 2 Soaked in 0.6mL of 5mol / L sulfuric acid solution for 2h, dried in 110℃ oven for 12h, calcined in air at 400℃ for 4h to obtain SO 4 2- / ZrO 2 , Where SO 4 2- The load is 22%.
[0025] Dissolve 1g reactant malic acid in 2g methanol and add SO 4 2- / ZrO 2 100mg. Heat to reflux at 60℃ for 2h, filter out the catalyst SO 4 2- / ZrO 2 , The reaction solution was rotated at 60° C. to remove methanol, and 1.14 g of dimethyl malate product was collected, with a yield of 95%.
[0026] The recovered catalyst was dried at 110° C. and used again in the above-mentioned esterification reaction of malic acid and methanol. The above-mentioned reaction conditions remained unchanged, and 1.14 g of dimethyl malate product was obtained with a yield of 95%.
Embodiment 2
[0028] Solid super acid catalyst SO 4 2- / ZrO 2 Preparation: Put 1g ZrO on the watch glass 2 Soaked in 0.6mL 10mol / L sulfuric acid solution for 4h, dried in 110℃ oven for 12h, calcined in air at 650℃ for 4h to obtain SO 4 2- / ZrO 2 , Where SO 4 2- The load is 58%.
[0029] Dissolve 1g reactant malic acid in 6g methanol and add SO 4 2- / ZrO 2 500mg. Heat to reflux at 60℃ for 4h, filter out the catalyst SO 4 2- / ZrO 2 , The reaction solution was rotated at 60° C. to remove methanol, and 1.18 g of dimethyl malate product was collected, with a yield of 98%.
[0030] The recovered catalyst was dried at 110°C and used in the above-mentioned malic acid and methanol esterification reaction, the above-mentioned reaction conditions remained unchanged, and the number of cycles was 2, 3, 4, and 5 times to obtain dimethyl malate product They are 1.18g, 1.18g, 1.16g, and 1.16g respectively. The yields were 98%, 98%, 97%, and 97%, respectively.
Embodiment 3
[0032] Solid super acid catalyst SO 4 2- / MoO 3 Preparation: Put 1g MoO on the watch glass 3 Soaked in 0.5mL of 5mol / L sulfuric acid solution for 2h, dried in 110℃ oven for 12h, calcined in air at 400℃ for 4h to obtain SO 4 2- / MoO 3 , Where SO 4 2- The load is 23%.
[0033] Dissolve 1g of reactant malic acid in 2g of ethanol and add SO 4 2- / MoO 3 100mg. Heat to reflux at 70℃ for 2h, filter out the catalyst SO 4 2- / MoO 3 , The reaction solution was rotated at 60°C to distill out ethanol, and 1.35 g of diethyl malate product was collected, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com