Synthesis method of quinocetone
A synthetic method and quinocetone technology, which is applied in the field of synthesis of veterinary drug quinocetone, can solve the problems of difficulty in obtaining high-yield qualified products and difficulties in purification, and achieve the effects of simple and convenient production process, excellent yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
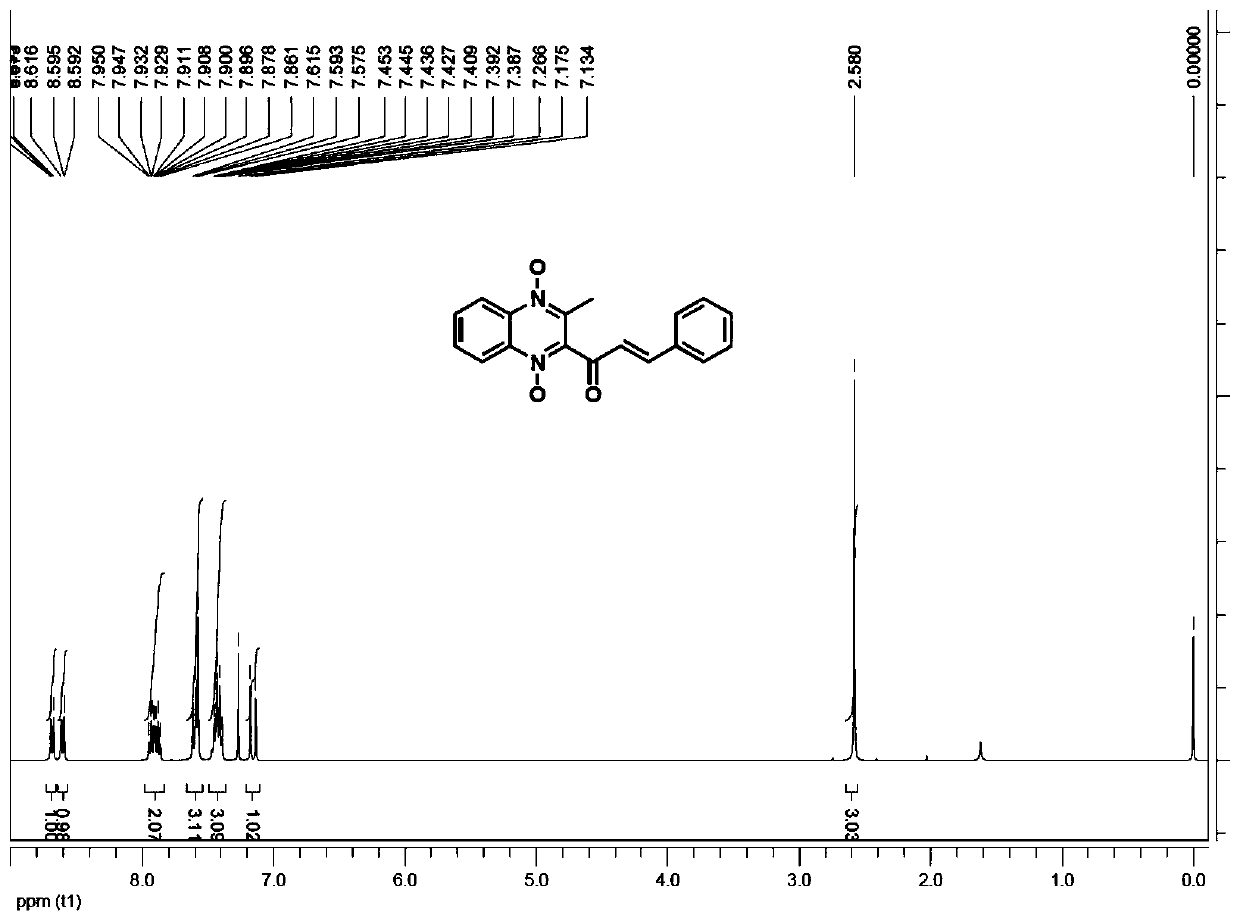
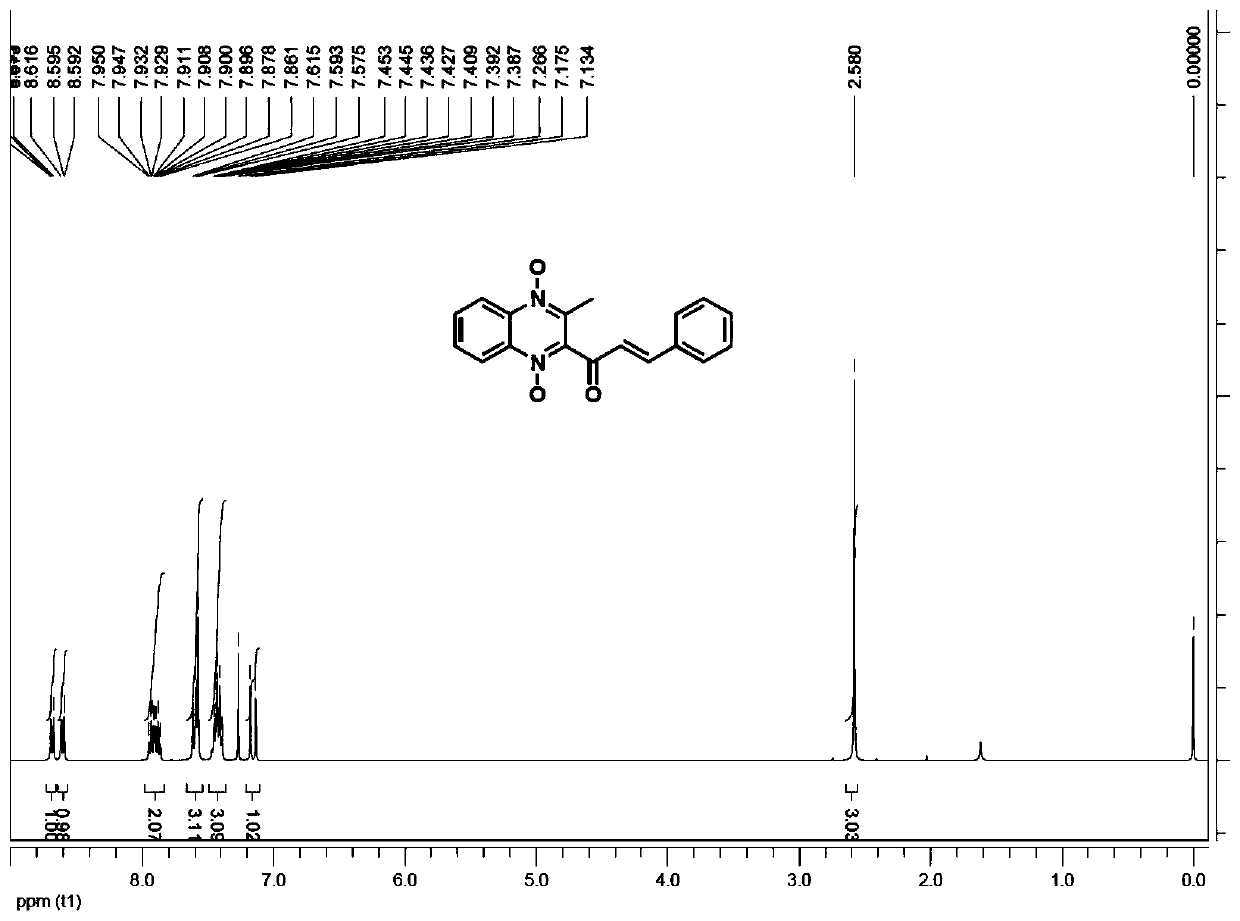
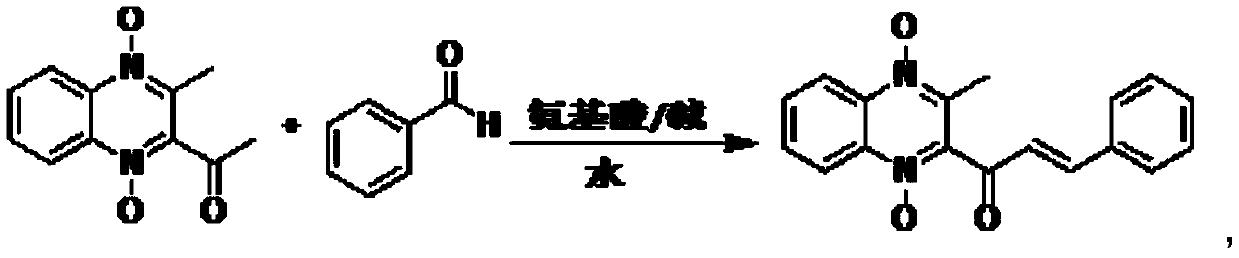
[0032] Weigh 1.2 g of valine, 1 g of 4-picoline, and 5 g of diethylene glycol, add them into a 250 ml reaction flask, add 80 ml of purified water, and stir to clarify the system.
[0033] Weighed 21.8 g of methaquine and 13.8 g of benzaldehyde respectively, added them to the above catalytic system, heated to 40° C. for 6 hours, and HPLC detected that the reaction of methaquine was complete, cooled, and suction filtered to obtain the crude quinocetone.
[0034] Add 60 ml of methanol to the crude quinocetone, stir for 1 hour, filter with suction, and dry in vacuum to obtain 27.6 g of the product with a yield of 90% and a purity of 98.2%.
Embodiment 2
[0036] Weigh 1 g of phenylalanine, 0.5 g of pyridine, and 2 g of triethylene glycol, add them into a 250 ml reaction flask, add 80 ml of pure water, and stir to clarify the system.
[0037] Weighed 21.8 g of methaquine and 12.8 g of benzaldehyde respectively, added them to the above catalytic system, heated to 40° C. and reacted for 4 hours, HPLC detected that the reaction of methaquine was complete, cooled, and suction filtered to obtain the crude quinocetone.
[0038] Add 80 ml of methanol to the crude quinocetone, stir for 1 hour, filter with suction, and dry in vacuum to obtain 28.1 g of the product with a yield of 92% and a purity of 98.1%.
Embodiment 3
[0040] Weigh 1.5 g of proline, 1.5 g of sodium carbonate, and 2 g of polyethylene glycol 400, add them into a 250 ml reaction flask, add 80 ml of purified water, and stir to clarify the system.
[0041] Weighed 21.8 g of methaquine and 11.8 g of benzaldehyde respectively, added them to the above catalytic system, heated to 25° C. for 5 hours, HPLC detected that the methaquine was completely reacted, cooled, and suction filtered to obtain the crude quinocetone.
[0042] Add 80 ml of methanol to the crude quinocetone, stir for 1 hour, filter with suction, and dry in vacuo to obtain 28.6 g of the product with a yield of 93% and a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com