A nickel phosphide catalyst for the preparation of 2,3,3,3-tetrafluoropropene by gas phase selective hydrodechlorination
A technology of selective hydrogenation and tetrafluoropropene, applied in physical/chemical process catalysts, dehydrohalogenation preparation, organic chemistry, etc., can solve the problems of increased reaction process costs, reduced selectivity of target products, etc., and achieve high temperature anti-sintering ability Good, low raw material cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
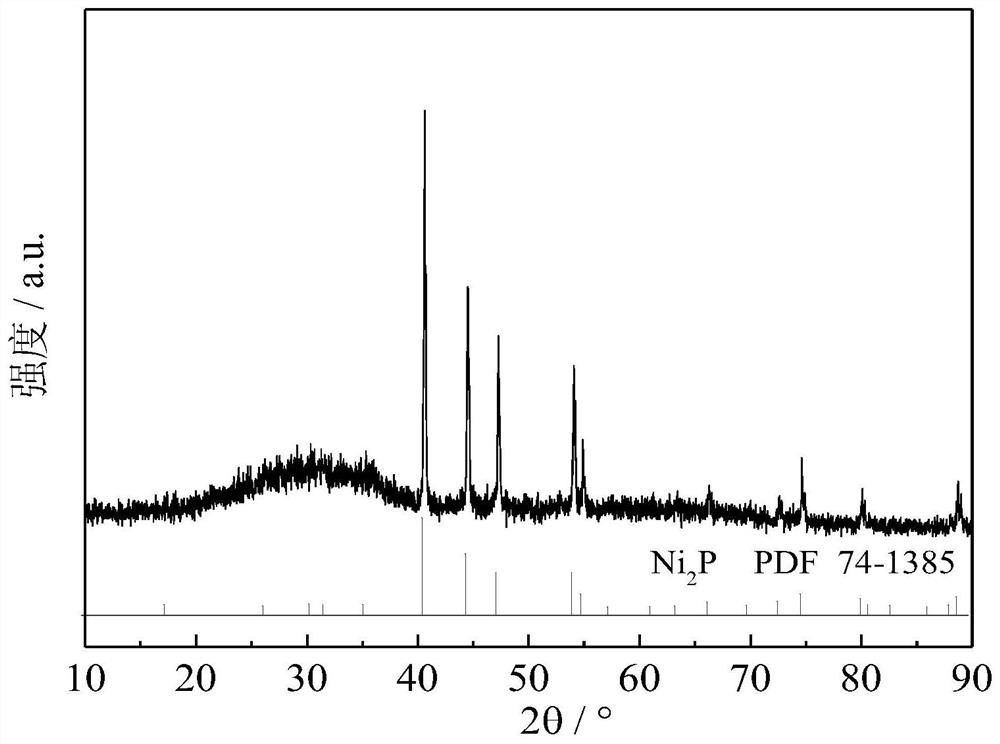
[0022] Embodiment 1: prepare Ni 2 P / oxide (fluoride, molecular sieve) catalyst
[0023] 9.48g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 3.45g diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 20 mL of deionized aqueous solution, and then the pH of the solution was adjusted to 2-3 with concentrated nitric acid to obtain a clear green solution. According to the loading of 0.5%, 5%, 10%, 20%, and 30%, the above solutions are impregnated onto a certain mass of oxides, fluorides, or molecular sieves, and then aged at room temperature for 12 hours, dried at 120 ° C for 12 hours to dry the water, and Calcined at 500°C for 3h to obtain the precursor of phosphate. The phosphide catalyst was prepared by in-situ temperature-programmed reduction method. The temperature programming step mainly includes two steps: (1) in H 2 Under the atmosphere (flow rate 150mL / min), the temperature was raised from room temperature to 120°C at 5°C / min, and kept at 120°C for 1h to remove...
Embodiment 2
[0024] Embodiment 2: prepare Ni 12 P 5 / activated carbon catalyst
[0025] 9.48g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) and 1.79g diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) was added to 20 mL of deionized aqueous solution, and then the pH of the solution was adjusted to 2-3 with concentrated nitric acid to obtain a clear green solution. According to the loads of 5%, 10%, 20%, and 30%, the above solutions were impregnated on a certain mass of activated carbon, and then aged at room temperature for 12 hours, dried at 120 ° C for 12 h, and roasted at 500 ° C for 3 h in a nitrogen atmosphere to obtain Phosphate precursor. The phosphide catalyst was prepared by in-situ temperature-programmed reduction method. The temperature programming step mainly includes two steps: (1) in H 2 Under the atmosphere (flow rate 150mL / min), the temperature was raised from room temperature to 120°C at 5°C / min, and kept at 120°C for 1h to remove the water adsorbed by the catalyst; (2) fro...
Embodiment 3
[0026] Example 3: Using the catalyst prepared by the method in Example 1 and 2, apply it to the gas phase selective hydrodechlorination of 2-chloro-1,1,1,2-tetrafluoropropane to prepare 2,3,3,3- In the reaction of tetrafluoropropene, after running for 8 hours, the reaction results are as follows:
[0027] Table 1 Conversion rate and product selectivity of HCFC-244bb on nickel phosphide catalysts with different loadings on different carriers for selective hydrodechlorination reaction.
[0028] serial number catalyst HCFC-244bb conversion rate / % HFO-1234yf selectivity / % 1 0.5wt.%Ni 2 P / SiO 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com