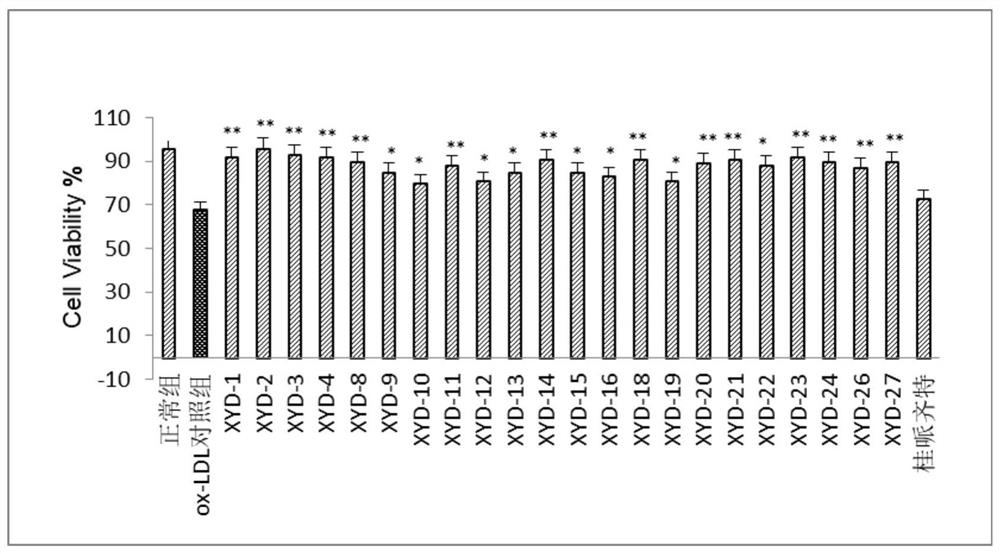
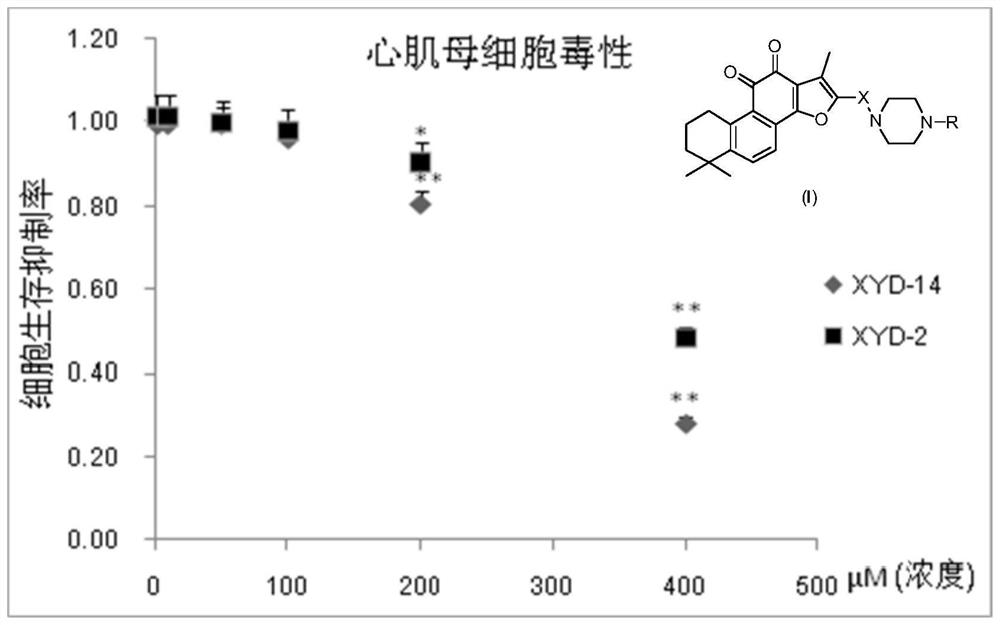
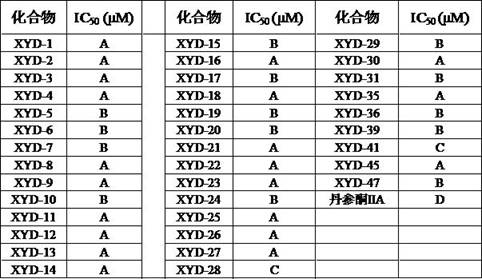
Tanshinone IIA piperazine compound and its preparation method and application
A compound, tanshinone technology, applied in the field of medicine, can solve the problem of unclear target mechanism, etc., and achieve the effect of strong calcium ion antagonism, good water solubility and lipid-water distribution coefficient, and good endothelial cell protection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Synthesis of Example 1 Compound XYD-1
[0081] Paraformaldehyde (15 mg, 0.5 mmol), zinc acetate (2 mg, 0.01 mmol), and 1-benzhydrylpiperazine (50 mg, 0.2 mmol) were added to a solution containing tanshinone IIA (59 mg, 0.2 mmol) in chloroform / Acetic acid solution (3 mL), and react overnight under reflux conditions. After the TLC detection reaction was complete, filter, and the gained filtrate was evaporated to dryness under reduced pressure and directly purified by silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =10:1~1:1), the compound XYD-1 of Example 1 (99 mg, yield 89%) was obtained. MS-ESI [M+H] + 559.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.62-7.57 (m, 2H), 7.30-7.23 (m, 10H), 4.21(s, 1H), 3.60 (s, 2H), 3.18 (t, J = 6.3 Hz, 2H), 2.72-2.38 (m, 8H), 2.24 (s,3H), 1.88-1.74 (m, 2H), 1.67-1.62 (m, 2H), 1.31 (s, 6H).
[0082] The preparation of Example 2 to Example 13 refers to the operation route of Example 1, using different substituted piperazines (commercially a...
Embodiment 14
[0087] The synthesis of embodiment 14 compound XYD-14
[0088] Tanshinone IIA formic acid (34 mg, 0.1 mmol) was added to the N - 1 mL of cyclopentylaminoethylpiperazine (20 mg, 0.1 mmol), EDCI (38 mg, 0.2 mmol) and HOBt (13 mg, 0.1 mmol) N , N - in dimethylformamide solution, and stirred at room temperature for 24 hours. After the reaction was completed, 5 mL of water was added to quench, then ethyl acetate (2×10 mL) was added to extract, washed with saturated brine, the obtained organic phase was dried over anhydrous sodium sulfate, concentrated and evaporated to dryness under reduced pressure, and subjected to silica gel column chromatography Purification (V 氯仿 :V 甲醇 =80:1~20:1), to obtain the compound XYD-14 of Example 14 (39 mg, yield 75%). MS-ESI [M+H] + 518.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.72-7.57 (m, 2H), 3.86-3.70 (m, 3H), 3.52-3.20 (m, 5H), 2.82-2.46 (m, 11H), 2.02-1.54 (m, 8H), 1.32-1.26 (m ,6H).
[0089] The preparation of Example 15 to Example 20 refe...
Embodiment 21
[0093] Example 21 Synthesis of Compound XYD-21
[0094] Tanshinone IIA acrylic acid (36 mg, 0.1 mmol) was added to the N - 1 mL of cyclopentylaminoethylpiperazine (20 mg, 0.1 mmol), DCC (41 mg, 0.2 mmol) and DMAP (24 mg, 0.2 mmol) N , N - dimethylformamide solution and stirred at room temperature for 24 hours. After the reaction was completed, 5 mL of water was added to quench, then ethyl acetate (10 mL) was added to extract, washed with saturated brine, the obtained organic phase was dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography (V 氯仿 :V 甲醇 =80:1~20:1), to obtain the compound XYD-21 of Example 21 (43 mg, yield 80%). MS-ESI [M+H] + 544.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.65-7.50 (m, 3H), 6.46 (d, J = 15.2 Hz, 1H),3.83-3.68 (m, 4H), 3.48-3.37 (m, 4H), 3.21-3.17 (m, 4H), 2.69-2.62 (m, 2H),2.40 (s, 3H), 2.01-1.74 (m, 6H), 1.65-1.61 (m, 2H), 1.31 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com