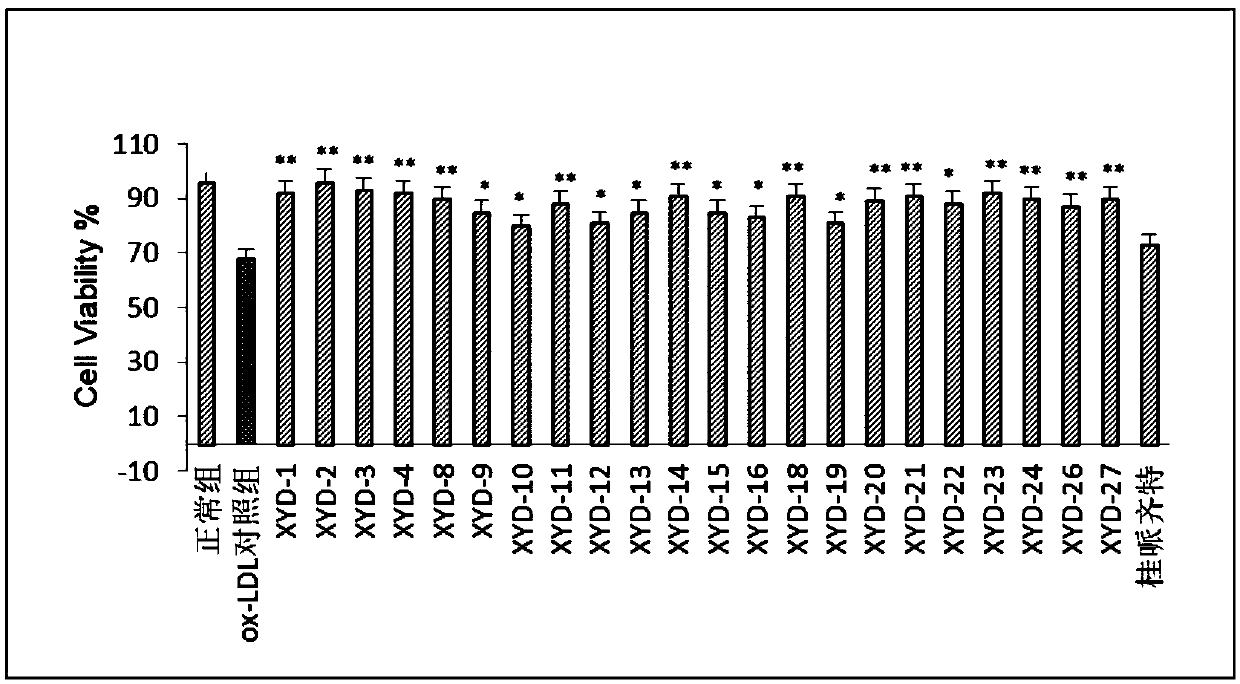
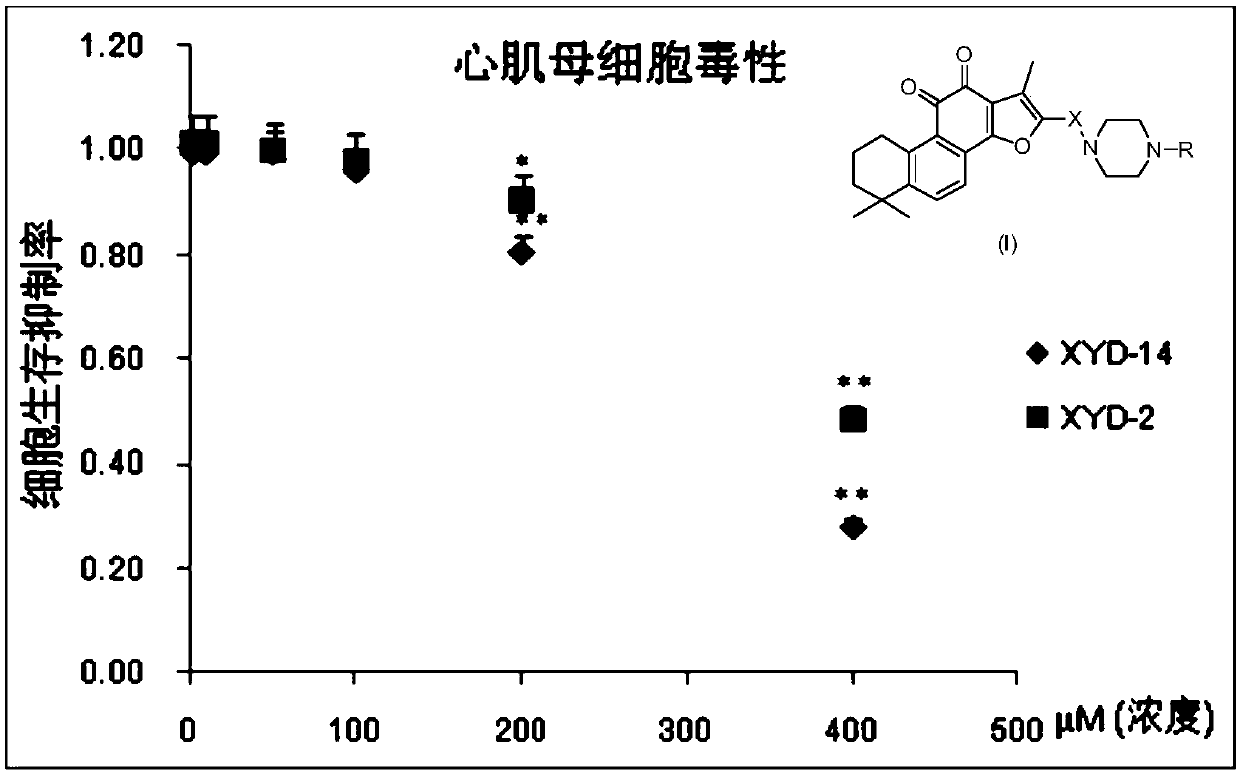
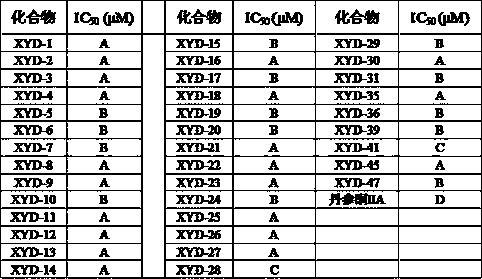
Tanshinone IIA piperazine compounds as well as preparation method and application thereof
A compound, tanshinone technology, applied in the field of medicine, can solve problems such as unclear target mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 Synthesis of compound XYD-1
[0081] Add paraformaldehyde (15 mg, 0.5 mmol), zinc acetate (2 mg, 0.01 mmol) and 1-benzyl piperazine (50 mg, 0.2 mmol) to the chloroform containing Tanshinone IIA (59 mg, 0.2 mmol) / Acetic acid solution (3 mL) and react overnight under reflux conditions. After the completion of the reaction detected by TLC, it was filtered. The filtrate obtained was evaporated to dryness under reduced pressure and purified directly by silica gel column chromatography (V Petroleum ether :V Ethyl acetate =10:1~1:1) to obtain compound XYD-1 of Example 1 (99 mg, yield 89%). MS-ESI [M+H] + 559.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.62-7.57 (m, 2H), 7.30-7.23 (m, 10H), 4.21(s, 1H), 3.60 (s, 2H), 3.18 (t, J = 6.3 Hz, 2H), 2.72-2.38 (m, 8H), 2.24 (s, 3H), 1.88-1.74 (m, 2H), 1.67-1.62 (m, 2H), 1.31 (s, 6H).
[0082] Preparation of Example 2 to Example 13 Refer to the operating route of Example 1, using different substituted piperazines (commercially available...
Embodiment 14
[0087] Example 14 Synthesis of Compound XYD-14
[0088] Add Tanshinone IIA formic acid (34 mg, 0.1 mmol) to the N -Cyclopentylethylpiperazine (20 mg, 0.1 mmol), EDCI (38 mg, 0.2 mmol) and HOBt (13 mg, 0.1 mmol) in 1 mL N , N -In the dimethylformamide solution, the reaction is stirred at room temperature for 24 hours. After the reaction is complete, add 5 mL of water to quench, then add ethyl acetate (2×10 mL) for extraction, wash with saturated brine, the resulting organic phase is dried over anhydrous sodium sulfate, concentrated under reduced pressure and evaporated to dryness, and subjected to silica gel column chromatography Purification (V Chloroform :V Methanol =80:1-20:1) to obtain compound XYD-14 of Example 14 (39 mg, yield 75%). MS-ESI[M+H] + 518.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.72-7.57 (m, 2H), 3.86-3.70 (m, 3H), 3.52-3.20 (m, 5H), 2.82-2.46 (m, 11H), 2.02-1.54 (m, 8H), 1.32-1.26 (m , 6H).
[0089] Preparation of Example 15 to Example 20 Refer to the operating rout...
Embodiment 21
[0093] Example 21 Synthesis of Compound XYD-21
[0094] Add Tanshinone IIA acrylic acid (36 mg, 0.1 mmol) to the N -Cyclopentylethylpiperazine (20 mg, 0.1 mmol), DCC (41 mg, 0.2 mmol) and DMAP (24 mg, 0.2 mmol) in 1 mL N , N -Dimethylformamide solution, and stir at room temperature for 24 hours. After the reaction was completed, it was quenched by adding 5 mL of water, then added with ethyl acetate (10 mL) for extraction, washed with saturated brine, the resulting organic phase was dried over anhydrous sodium sulfate, evaporated to dryness under reduced pressure, and purified by silica gel column chromatography (V Chloroform :V Methanol =80:1-20:1) to obtain compound XYD-21 of Example 21 (43 mg, yield 80%). MS-ESI [M+H] + 544.3; 1 H NMR (400 MHz, CDCl 3 ) δ 7.65-7.50 (m, 3H), 6.46 (d, J = 15.2 Hz, 1H), 3.83-3.68 (m, 4H), 3.48-3.37 (m, 4H), 3.21-3.17 (m, 4H), 2.69-2.62 (m, 2H), 2.40 (s, 3H), 2.01-1.74 (m, 6H), 1.65-1.61 (m, 2H), 1.31 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com