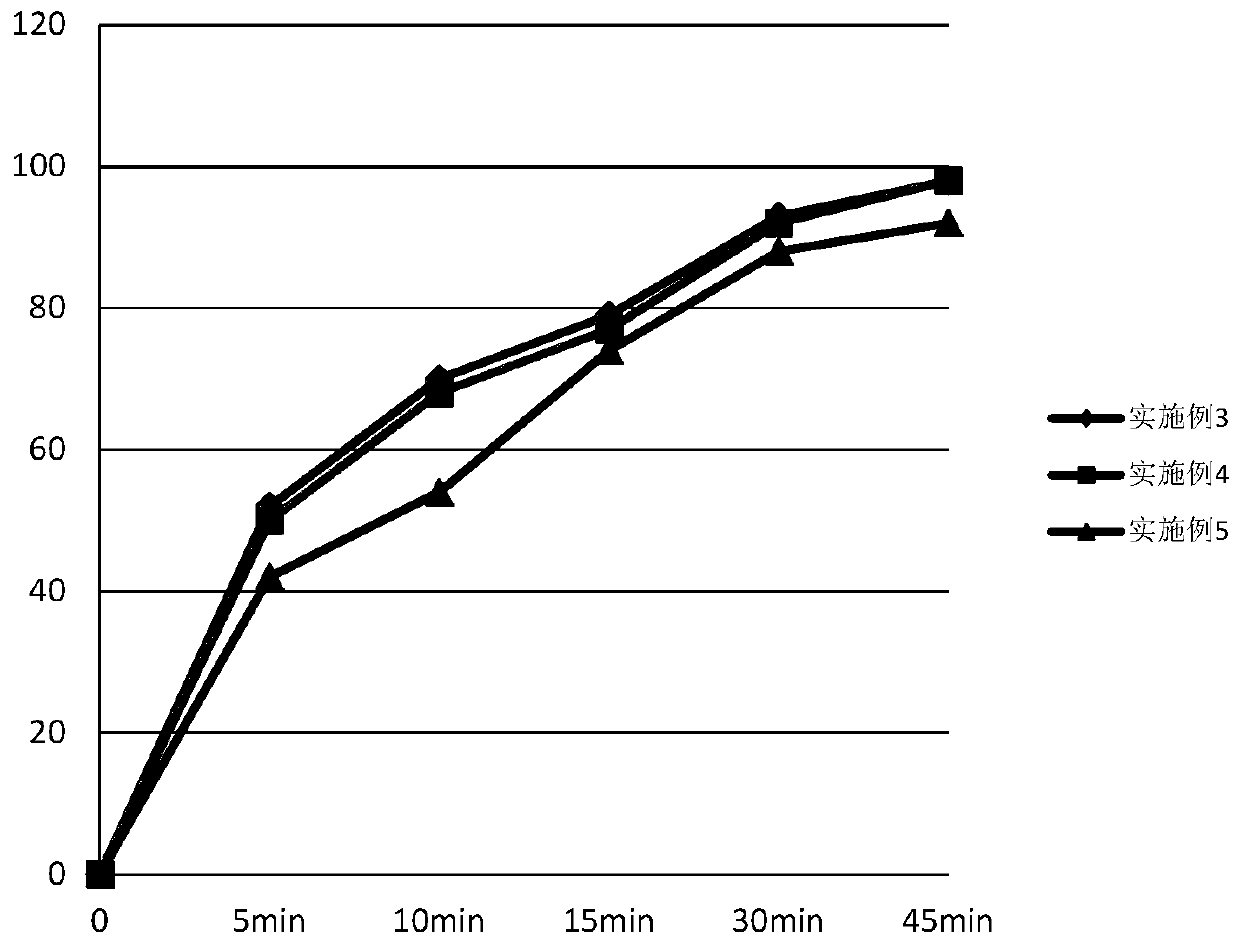
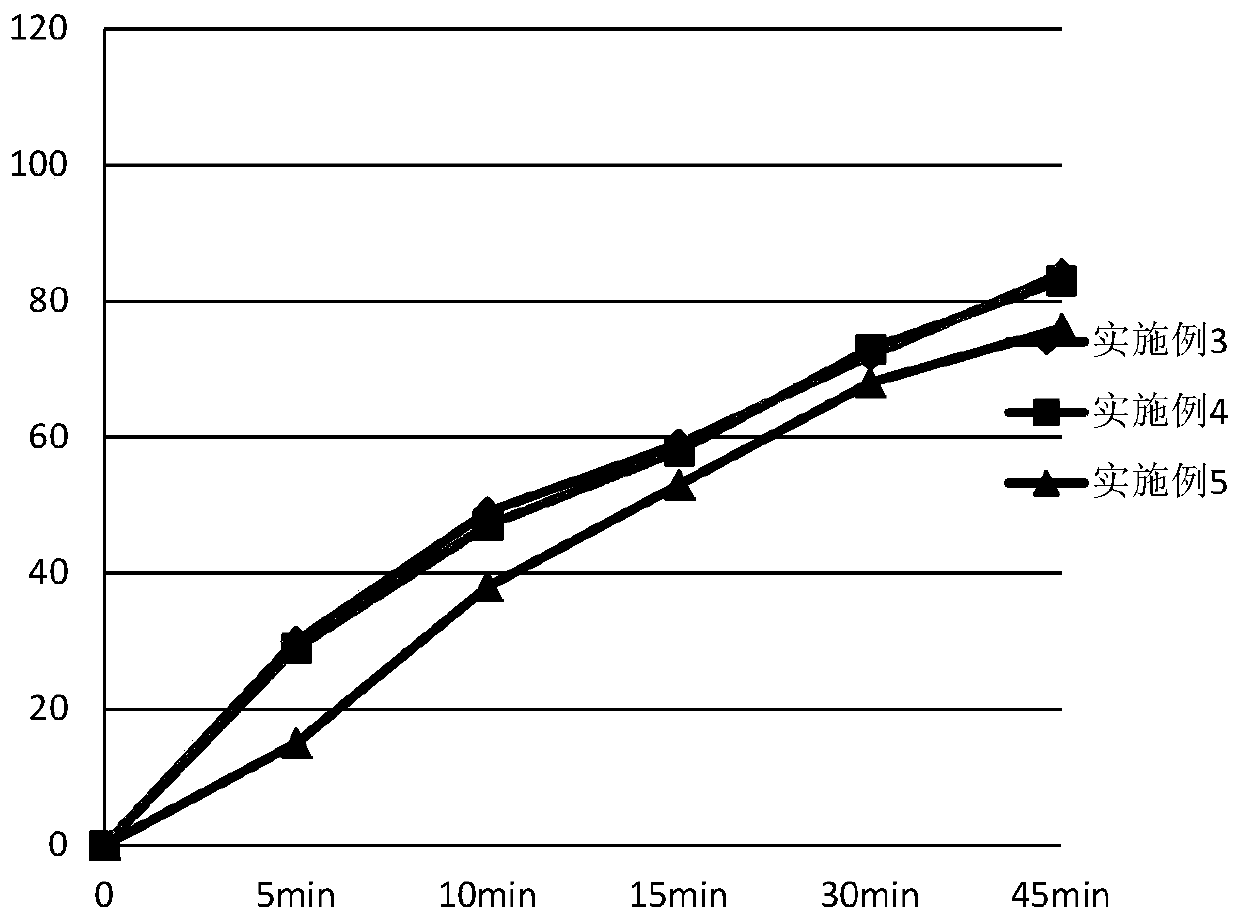
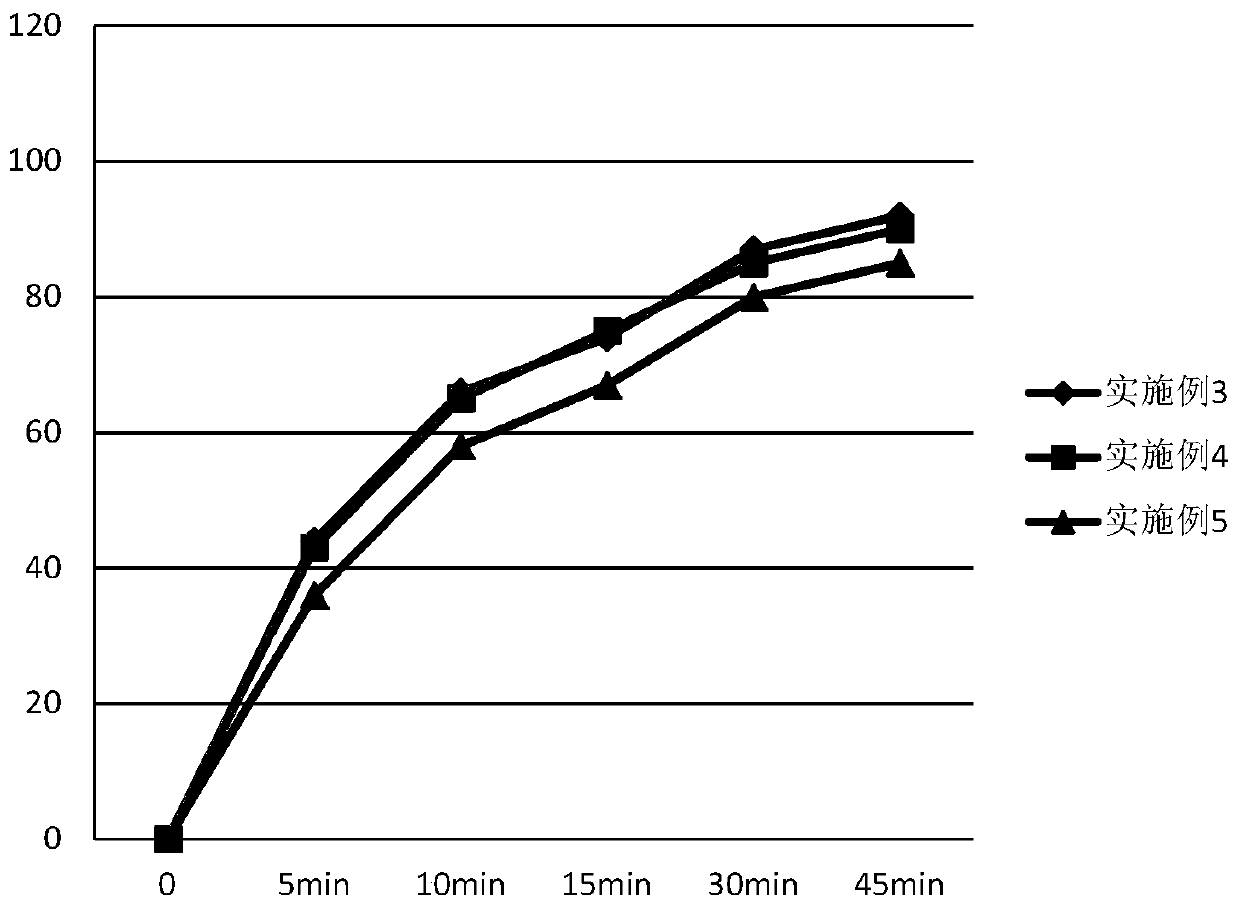
Ambrisentan oral tablets and preparation method thereof
An ambrisentan tablet and a technology for ambrisentan, which are applied in the field of ambrisentan tablets and their production, can solve the problems of affecting drug safety and effectiveness, large differences in dissolution between tablets, large differences in dissolution and the like, and achieve dissolution Small variance, high dissolution, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1 particle size investigation
[0053] Table 1
[0054]
[0055]
[0056] Conclusion: By investigating the angle of repose, difference in tablet weight, mixing uniformity, content uniformity of plain tablet and dissolution rate of plain tablet, under the particle size of the present invention, the difference in tablet weight of the obtained plain tablet is small, and has good content Uniformity and Mix Uniformity.
Embodiment 2
[0057] Embodiment 2 hardness is investigated
[0058] Table 2
[0059]
[0060]Conclusion: Through the investigation of the appearance and friability of plain tablets, when the hardness range of ambrisentan tablets is above 6.00, the obtained tablets are complete and smooth, with uniform color and no missing corners or broken.
Embodiment 3
[0062] Tablet prescription:
[0063]
[0064]
[0065] Preparation Process:
[0066] (1) Pretreatment of raw and auxiliary materials: sieve ambrisentan, control d (0.9) to 24.9um, lactose and microcrystalline cellulose control d (0.9) to 43.9um;
[0067] (2) Put the prescribed amount of ambrisentan and half of the prescribed amount of lactose into the hopper mixer, and mix for 10 minutes;
[0068] (3) adding the intermediate material obtained in (2) into the granulator to carry out granulation;
[0069] (4) Add the sized intermediate material and the other half of the prescription amount of lactose into the hopper mixer, and mix for 10 minutes;
[0070] (5) Add microcrystalline cellulose and croscarmellose sodium into the hopper mixer in the prescribed amount, and mix for 15 minutes;
[0071] (6) Add the magnesium stearate of recipe quantity in the hopper mixer, mix 10min;
[0072] (7) Add the material obtained in (6) into a rotary tablet press for tableting, the ave...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com