Compound, preparation method and uses thereof
A technology for compounds and compositions, applied in the fields of compounds and their preparation and use, can solve problems such as hidden safety hazards and ineffectiveness in disease development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
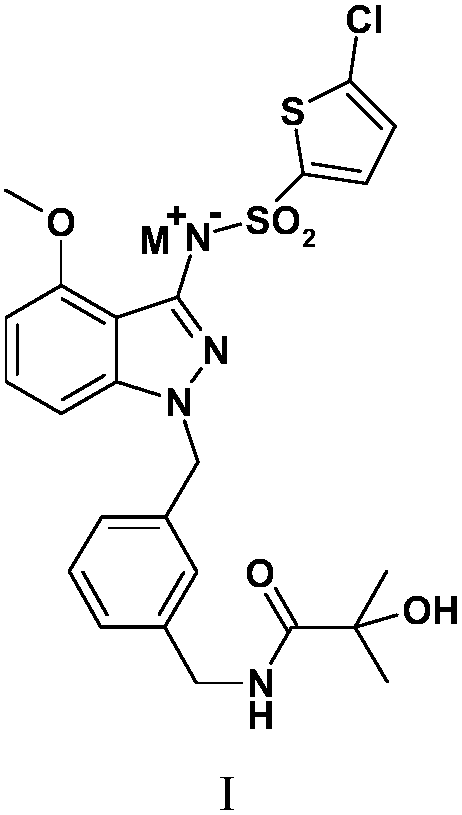
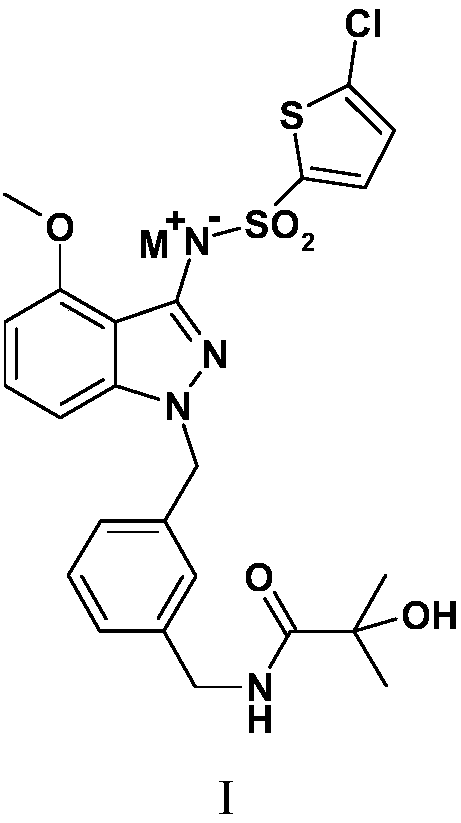
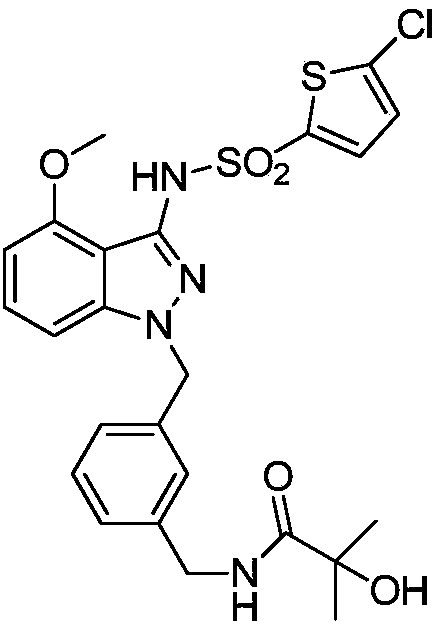
[0058] Example 1N-[(3-{[3-{[(5-chloro-2-thienyl)sulfonyl]amino}-4-(methoxy)-1H-indazol-1-yl]methyl} Preparation of phenyl)methyl]-2-hydroxy-2-methylpropionamide
[0059] Preparation of N-[(3-{[3-{[(5-chloro-2-thienyl)sulfonyl]amino}-4-(methoxy)-1H-indazol-1-yl of the following formula compound 6 ]methyl}phenyl)methyl]-2-hydroxy-2-methylpropionamide
[0060]
[0061] Step 1 Preparation of intermediate compound 1 4-(methoxy)-1H-indazol-3-amine
[0062]
[0063] 6-Fluoro-2methoxybenzonitrile (50g, 0.33mol) and hydrazine hydrate (50g, 0.99mol) were dissolved in 400ml of n-butanol, and refluxed for 20h under N2 protection. After the solution was cooled, 400ml of water was added and stirred evenly, the organic layer was separated, and the solid precipitated in the aqueous phase was collected. The solvent in the organic layer was removed under reduced pressure, and the residue was mixed with the aqueous phase and the precipitated solid in the aqueous phase, and extracted wit...
Embodiment 4
[0087] Embodiment 4 solubility test
[0088] The solubility of the compounds was determined according to the method established in the second part of the Pharmacopoeia of the 2010 edition. Accurately weigh the compound 6 prepared in Example 1, N-[(3-{[3-{[(5-chloro-2-thienyl)sulfonyl]amino}-4-(methoxy)-1H -Indazol-1-yl]methyl}phenyl)methyl]-2-hydroxy-2-methylpropionamide 10, 100, 1000 mg each, placed in a 500 ml volumetric flask at 25°C±2°C Add water to dilute to the mark, shake vigorously for 30 seconds every 5 minutes, observe the dissolution within 30 minutes, only 10 mg of the sample has no visible solute, and its solubility in water is 0.02 mg / ml.
[0089] Accurately weigh the formula II compound N-[(3-{[3-{[(5-chloro-2-thienyl)sulfonyl]amino}-4-(methoxy)-1H prepared in Example 2 -Indazol-1-yl]methyl}phenyl)methyl]-2-hydroxy-2-methylpropionamide sodium salt 10, 100, 1000, 2000 mg each, placed at 25°C±2°C In a 2ml volumetric flask, add water to dilute to the mark, shake...
Embodiment 5
[0095] Example 5 In vitro CCR4 antagonistic activity test
[0096] Antagonist potency was determined by radioligand [35S]-GTPyS competition assay. Briefly, CHO membranes expressing CCR4 were homogenized through a 23G needle. These membranes were then attached to WGA-coated LeadseekerSPA beads in assay buffer (20 mM HEPES, 10 mM MgCl, 100 mM NaCl, 0.05% BSA, 40 ug / ml saponin, pH adjusted to 7.4 using KOH 5M) to generate 3 μg / well final assay concentration (FAC) of membrane and 250 μg / well of FAC bead solution. After 60 min pre-coupling on ice, GDP was added to give 4.4uM FAC. [35S]-GTPyS prepared in assay buffer was then added to the bead / membrane solution to obtain 0.33 nM FAC. Addition of human MDCs to the bead / membrane / [35S]-GTPyS suspension resulted in FACs exhibiting 80% (EC80) of the maximal agonist response. The bead / membrane / [35S]-GTPyS / agonist suspension was dispensed into white Greiner polypropylene 384-well plates (45[mu]l / well) containing 0.5[mu]l of compound. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com