Specific double-photon imaging fluorescent probe as well as preparation method and application
A fluorescent probe and detection method technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of inability to real-time, in situ imaging detection, poor selectivity, etc., and achieve good two-photon properties, cell damage, etc. Small, deep penetration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
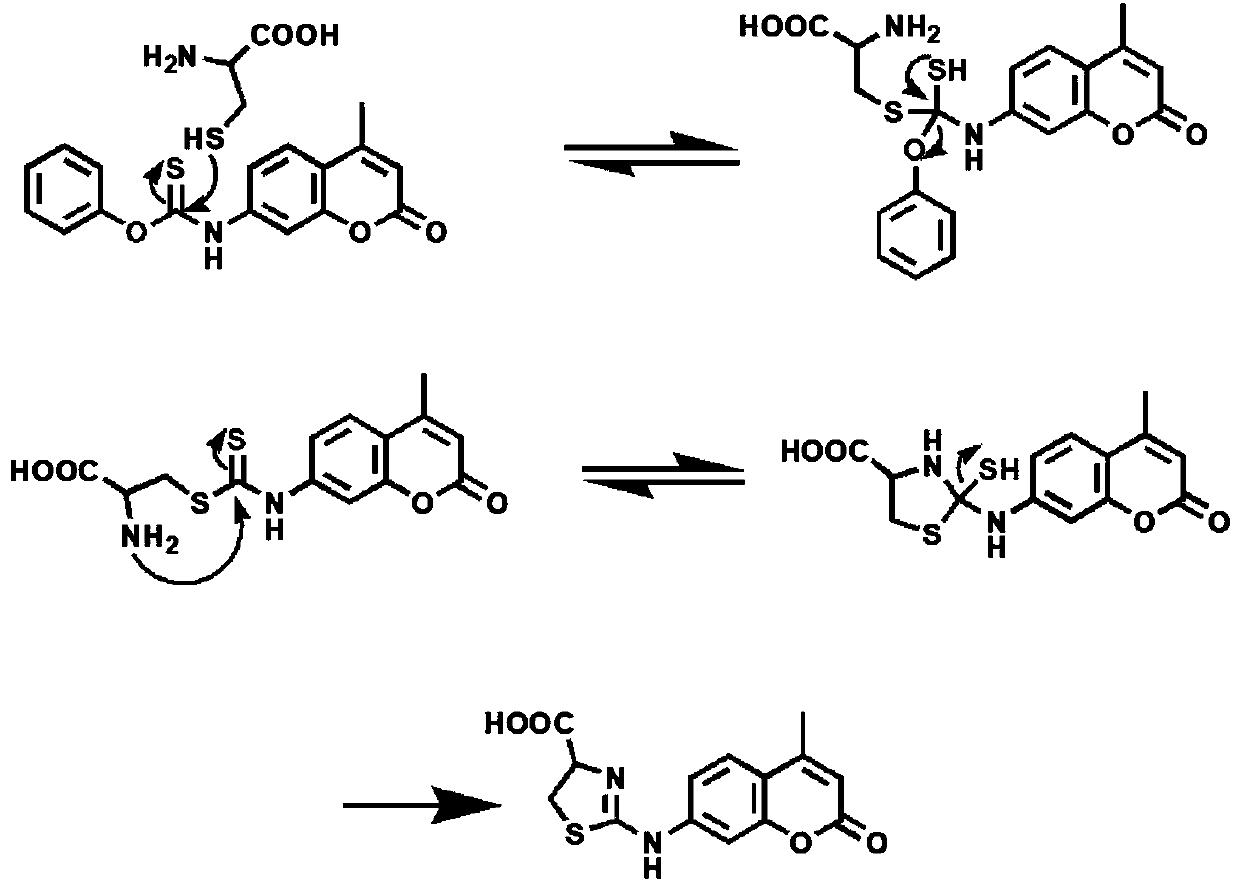
[0052] Synthesis of Fluorescent Probes (TCS)
[0053] Coumarin 120 (0.176 g, 1.0 mmol) was first dissolved in THF (10 mL) under nitrogen protection, and then phenylthiochloroformate (135 μL, 1.0 mmol) was added. Next the mixture was stirred at 28°C under nitrogen for 12 hours. After the reaction was complete, the mixture was concentrated in vacuo. Purify by thin-plate chromatography, the eluent is ethyl acetate:cyclohexane=2:1, to obtain a light yellow solid, namely the fluorescent probe, which is denoted as TCS. Finally, TCS was dissolved in Tris buffer (1% DMSO, pH=7.4) for further characterization and performance evaluation.
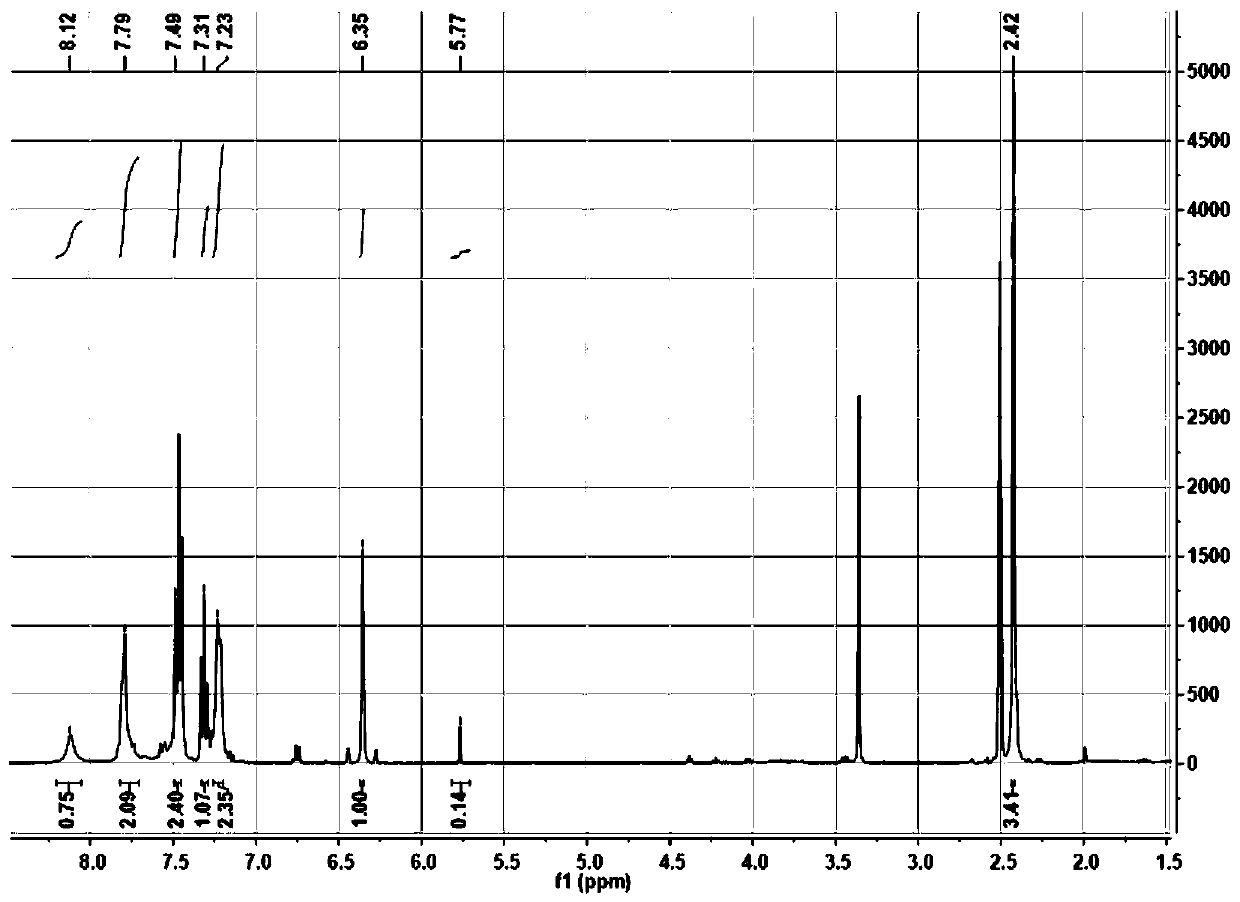
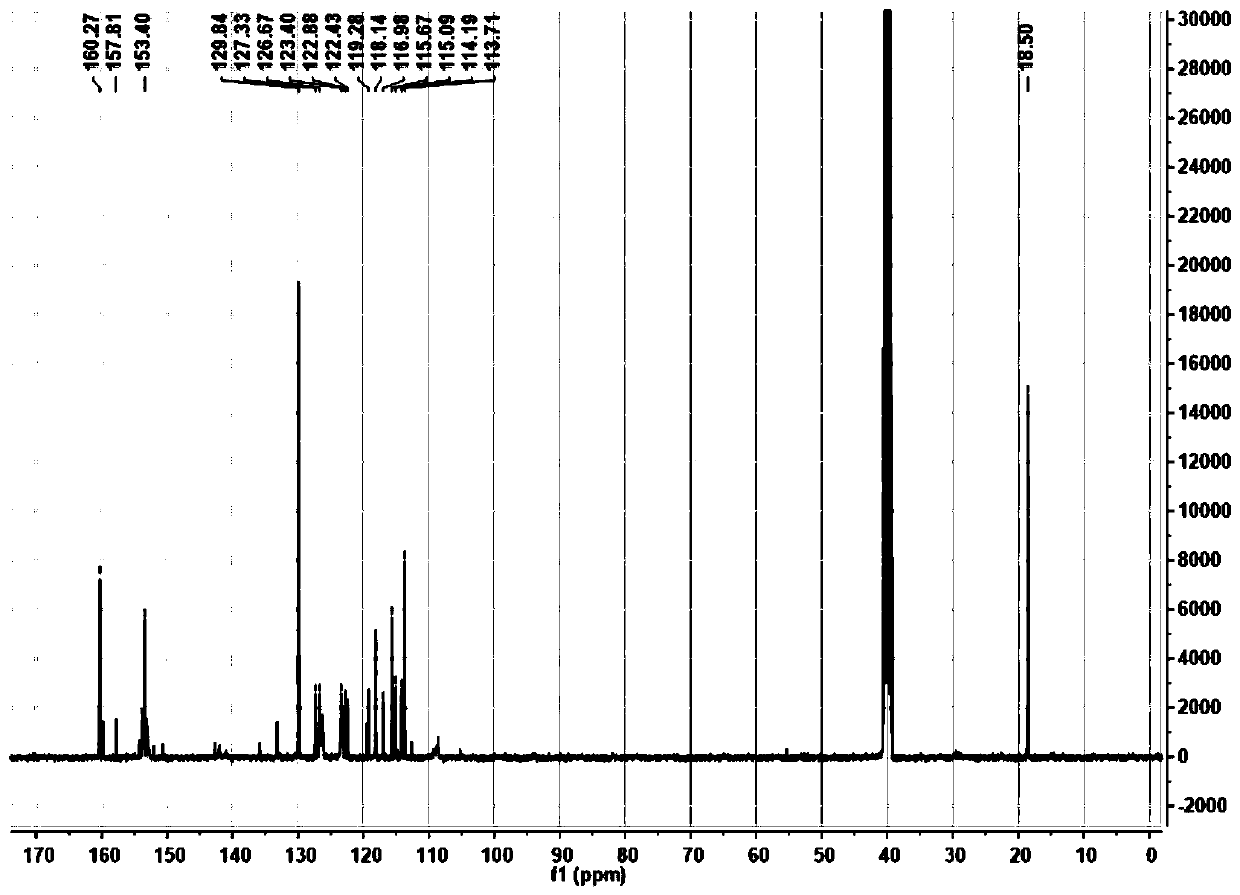
[0054] Mass Spectrometry and NMR Characterization of TCS
[0055] HRMS(ESI)m / z calcd.for C 17 h 13 NO 3 S[M+H] + calculated 312.0688, found 312.0670.NMRdata: 1 HNMR (400MHz, d 6 -DMSO), figure 1 Shown: δ8.12(s,1H),7.79(d,2H),7.49(t,2H),7.31(t,1H),7.23(d,2H),6.35(s,1H),5.77(s ,1H),2.42(s,3H). 13 C NMR (101MHz, d 6 -DMSO), figure 2 Shown:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com