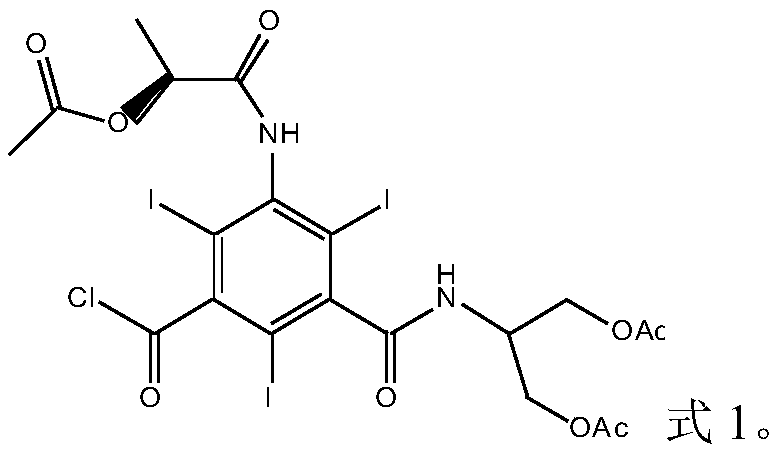
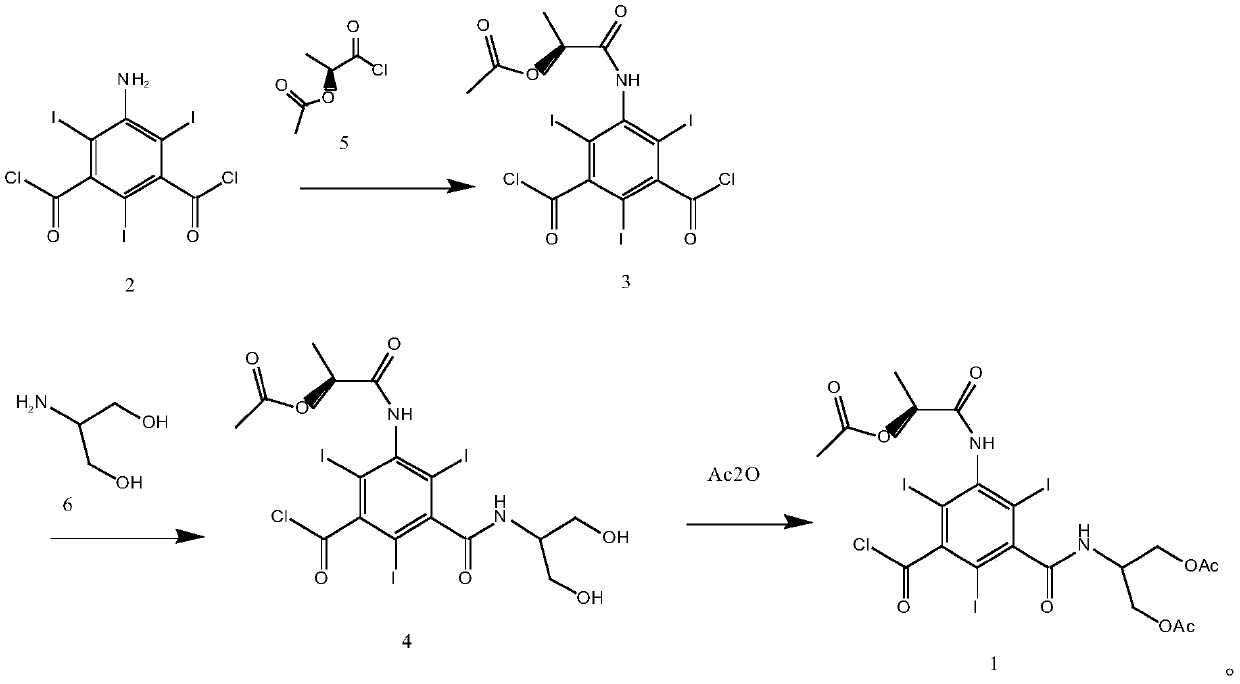
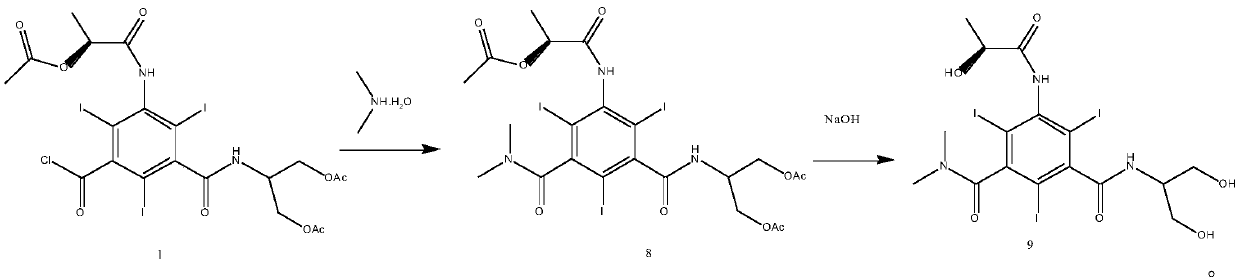
5-Amino-2,4,6-triiodisophthaloyl acid dichloride derivative and application thereof in synthesis of iopamidol impurities
A technology of triiodoisopeptidyl chloride derivatives and amino groups, which is applied in the field of medicine and can solve problems such as affecting product quality, difficult to control, and residual iopamidol products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthesis of formula 1 compound
[0052] (1) Synthesis of 5-[(2(S)-2-acetoxypropionyl)amino]-2,4,6-triiodo-1,3-phthaloyl chloride (compound of formula 3)
[0053] According to the molar ratio of 5-amino-2,4,6-triiodo-1,3-phthaloyl chloride to 2(S)-2-acetoxypropionyl chloride is 1:2, 30g of 5-amino-2, Put 4,6-triiodo-1,3-phthaloyl chloride in a 250ml reaction flask, add 60mL N,N-dimethylacetamide under nitrogen protection, stir at room temperature until completely dissolved, and cool down to 0-5°C And keep it warm, slowly add 15g of 2(S)-2-acetoxypropionyl chloride dropwise, after the dropwise addition, naturally raise the temperature to 15-20°C and keep it warm for 30-40 hours, add 150mL of dichloromethane, stir for 3-5 minutes, Wash three times with 300 mL of water each time, then wash twice with 200 mL of saturated brine each time, separate the organic phase, concentrate to dryness under reduced pressure, add 90 mL of isopropanol to stir and crystal...
Embodiment 2
[0059] Embodiment 2: the synthesis of iopamidol impurity D
[0060] According to 1-[2-hydroxyacetyl-1-(hydroxyacetylmethyl)ethyl]-3-[benzoyl chloride]-5-[(2(S)-2-acetoxypropionyl)amino]- The molar ratio of 2,4,6-triiodo-benzamide to sodium hydroxide is 1:5, and 15g of 1-[2-hydroxyacetyl-1-(hydroxyacetylmethyl)ethyl]-3-[benzyl Acyl chloride]-5-[(2(S)-2-acetoxypropionyl)amino]-2,4,6-triiodo-benzamide was put into a 250mL reaction bottle, and 60mL of dichloromethane was added to stir at room temperature to dissolve. Then add 60mL of pure water, add 17.7mL of 20% sodium hydroxide dropwise, and react at 20-30°C after the dropwise addition, and follow up and detect the completion of the reaction by HPLC, adjust the pH to 6-7 with 10% hydrochloric acid, and separate the layers to obtain the water layer. After desalting with cation resin and anion resin, the impurity D was obtained with a yield of 70% and a purity of 97.4% by HPLC.
Embodiment 3
[0061] Embodiment 3: the synthesis of iopamidol impurity F
[0062] (1) According to the molar ratio of the compound of formula 1 to triethylamine and dimethylamine 1:1.2:1.6, add 18 g of the compound of formula 1, add 3.3 mL of triethylamine in 20 mL of N,N-dimethylacetamide solution, Cool down in an ice bath, keep warm at 0-10°C, add 3.6g of 40% dimethylamine aqueous solution dropwise, after the drop is complete, heat up to 20-30°C to react, HPLC detects that the reaction is complete, filter, and the filtrate is concentrated under reduced pressure, then add 30mL of isopropanol, and reflux After 2-4 hours, cool down to 20-30°C, slowly add 60mL of acetone dropwise, stir at 20-30°C for 1-2 hours, filter, and dry to obtain 12g of N-[N,N′-dimethyl]-N′-[ 2-Glyoacetyl-1-(hydroxyacetylmethyl)ethyl]-5-[(2(S)-2-acetoxypropionyl)amino]-2,4,6-triiodo-1,3 - Phthalamides.
[0063] (2) According to N-[N,N'-dimethyl]-N'-[2-glycolyl-1-(glycolylmethyl)ethyl]-5-[(2(S)-2- The molar ratio of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com