Bisphenol hydroxyl monomer containing anthryl group and synthesis method and application thereof
A technology of anthracenyl group and bisphenol hydroxyl group, which is applied in the field of bisphenol hydroxyl monomer and its synthesis, can solve the problems of unbearable conditions for small functional molecules, few studies on post-functionalization, and incomplete removal of catalysts, etc., to achieve Good application prospects, high product yield, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
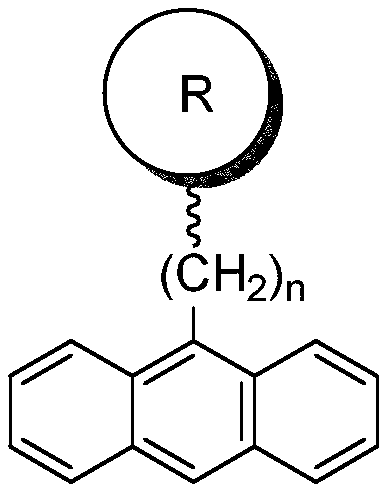
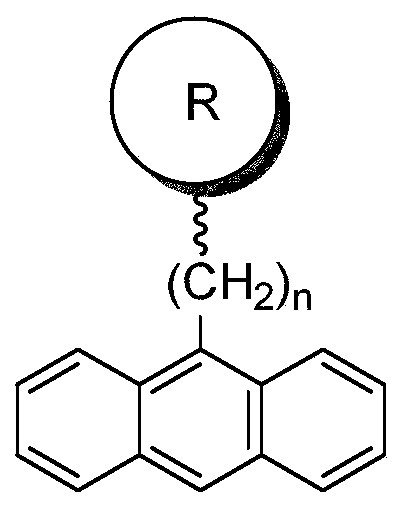
[0038] This embodiment provides a kind of bisphenol hydroxyl monomer containing anthracenyl group, and its specific structure is as follows:
[0039]
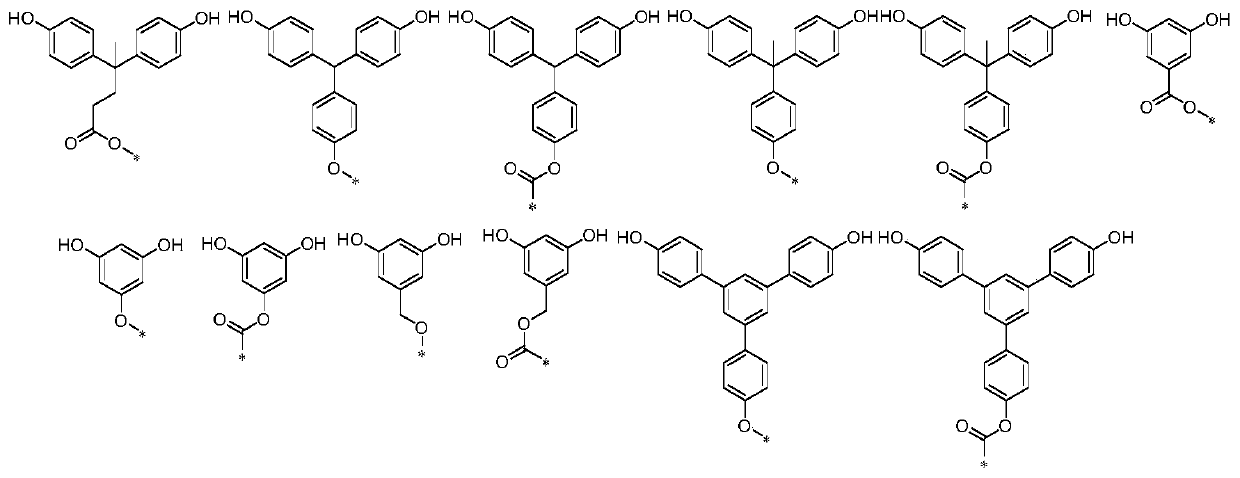
[0040] Also provide the synthetic method of above-mentioned compound, specifically comprise:
[0041]
[0042] Put 10mmol of bisphenolic acid, 12.5mmol of potassium bicarbonate, and 30ml of DMF into a 50ml single-necked flask, heat to 80°C for 30min, continue to add 10mmol of 9-bromomethylanthracene for 2h, and distill off excess Solvent, the resulting viscous product was dissolved in ethyl acetate, then washed 2-3 times with distilled water, the organic phases were combined and dried, the solvent was distilled off under reduced pressure, and the target product was obtained by silica gel column chromatography. Yield 84%.
[0043] 1 H NMR (400MHz, Acetone): δ8.62(s, 1H), 8.40(d, J=8.9Hz, 2H), 8.10(d, J=7.5Hz, 4H), 7.65–7.58(m, 2H), 7.56–7.47(m,2H), 6.98(d,J=8.2Hz,4H),6.70(d,J=8.1Hz,4H),6.14(s,2H),2.41–2.33 (m,2H),2.13 ...
Embodiment 2
[0046] This embodiment provides a kind of bisphenol hydroxyl monomer containing anthracenyl group, and its specific structure is as follows:
[0047]
[0048] Also provide the synthetic method of above-mentioned compound, specifically comprise:
[0049]
[0050] Put 10mmol of 3,5-dihydroxybenzoic acid, 12.5mmol of potassium bicarbonate, and 30ml of DMF solvent into a 50ml single-necked flask, heat to 80°C for 30min, continue to add 10mmol of 9-bromomethylanthracene for 2h, reduce The excess solvent was distilled off under pressure, and the obtained viscous product was dissolved in ethyl acetate, then washed 2-3 times with distilled water, the organic phases were combined and dried, the solvent was distilled off under reduced pressure, and the target product was obtained by silica gel column chromatography. Yield 87%.
Embodiment 3
[0052] This embodiment provides a kind of bisphenol hydroxyl monomer containing anthracenyl group, and its specific structure is as follows:
[0053]
[0054] Also provide the synthetic method of above-mentioned compound, specifically comprise:
[0055]
[0056] Put 10mmol of 1,1,1-tris(4-hydroxyphenyl)ethane, 12.5mmol of potassium carbonate, and 30ml of DMF solvent into a 50ml one-necked flask, heat to 80°C for 30min, and continue to add 10mmol of 9-bromo Methyl anthracene was reacted for 2 hours, the excess solvent was distilled off under reduced pressure, and the obtained viscous product was dissolved in ethyl acetate, then washed 2-3 times with distilled water, the organic phases were combined, dried, and the solvent was removed by distillation under reduced pressure, and silica gel column chromatography obtain the target product. Yield 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com