Synthesis method of fluticasone propionate impurities
A technology of fluticasone propionate and synthesis method, applied in the direction of steroids, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
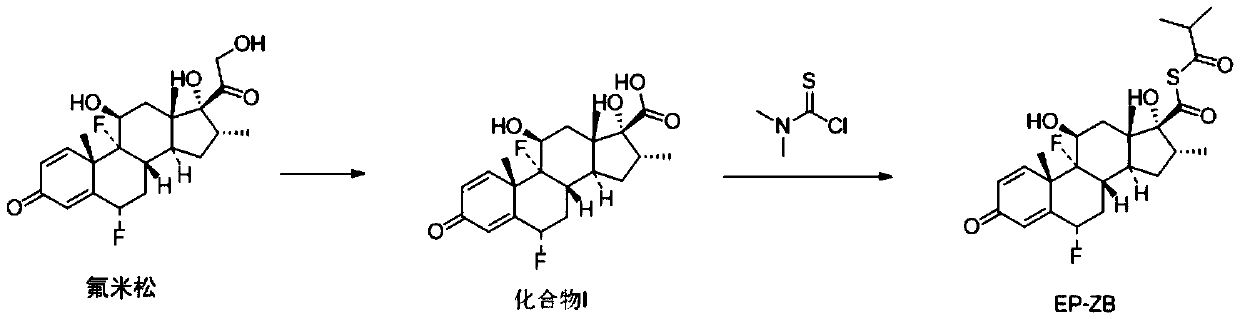
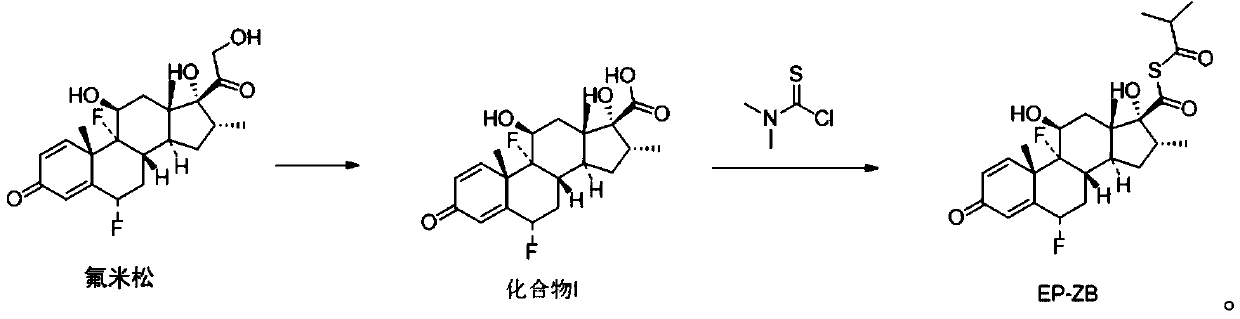
[0036] (1) Synthesis of compound I
[0037]
[0038] Take a 1.0L three-necked reaction flask, add 30.0g of flumetasone and 165mL of tetrahydrofuran, stir to dissolve, weigh 25.0g of periodic acid and add 225mL of water to dissolve, slowly add the periodic acid solution into the reaction flask, after adding the sample, control the temperature React at 0-20°C for 2 hours; after the reaction is completed, add 225mL of purified water to the reaction solution, control the temperature to 0-20°C, carry out stirring and crystallization for 40min, suction filtration, filter cake rinse with 90mL water, suction filtration, filter cake After drying at 40°C, 28.72 g of off-white solid, ie Compound I, was obtained with a yield of 99.12% and a purity of 98%. Wherein, the periodic acid concentration (mass fraction) is 10%.
[0039] (2) Synthetic impurity EP-ZB
[0040]
[0041]Take a 250mL three-necked reaction flask, add 2.01g of compound I and 25mL of acetone, control the temperatur...
Embodiment 2
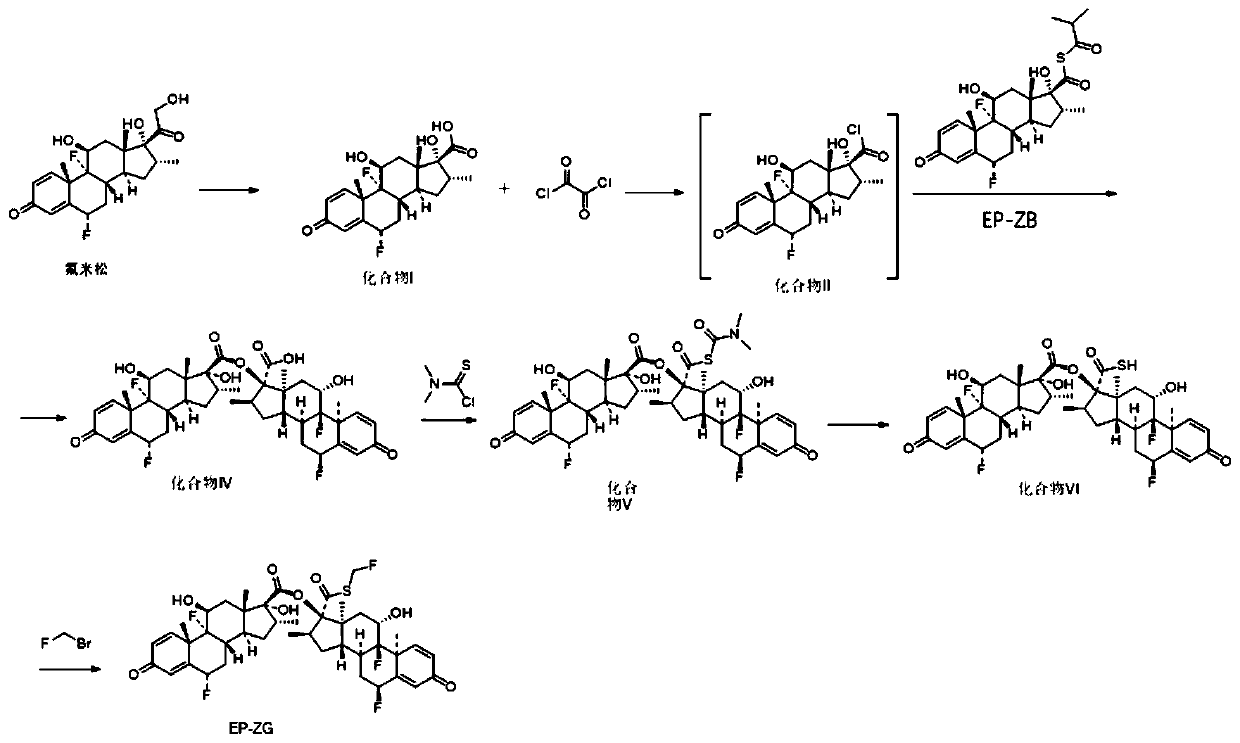
[0043] (1) Synthesis of compound I
[0044]
[0045] Take a 1.0L three-necked reaction flask, add 30.0g of flumetasone and 165mL of tetrahydrofuran, stir to dissolve, weigh 25.0g of periodic acid and add 225mL of water to dissolve, slowly add the periodic acid solution into the reaction flask, after adding the sample, control the temperature React at 0-20°C for 2 hours; after the reaction is completed, add 225mL of purified water to the reaction solution, control the temperature to 0-20°C, carry out stirring and crystallization for 40min, suction filtration, filter cake rinse with 90mL water, suction filtration, filter cake After drying at 40°C, 28.72 g of off-white solid, ie Compound I, was obtained with a yield of 99.12% and a purity of 98%.
[0046] (2) Synthesis of Compound IV
[0047]
[0048] Take a 250mL three-necked reaction flask, add 8.02g compound I and 50mL dichloromethane, cool down to 0-10°C, add 0.3mL N,N-dimethylformamide and stir for 20min, then control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com