A kind of preparation method of 3-indolated cyclohexenone compound
A technology of cyclohexenone and compound is applied in the field of preparation of indoleated cyclohexenone derivatives, and achieves the effects of simple reaction steps, mild reaction conditions and improved total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
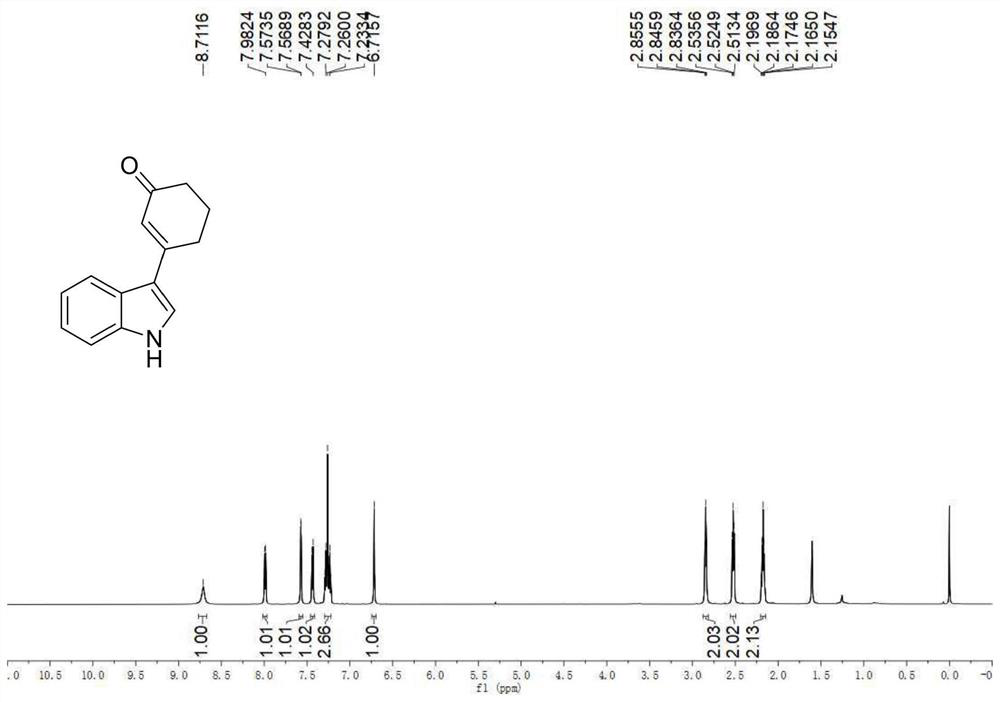
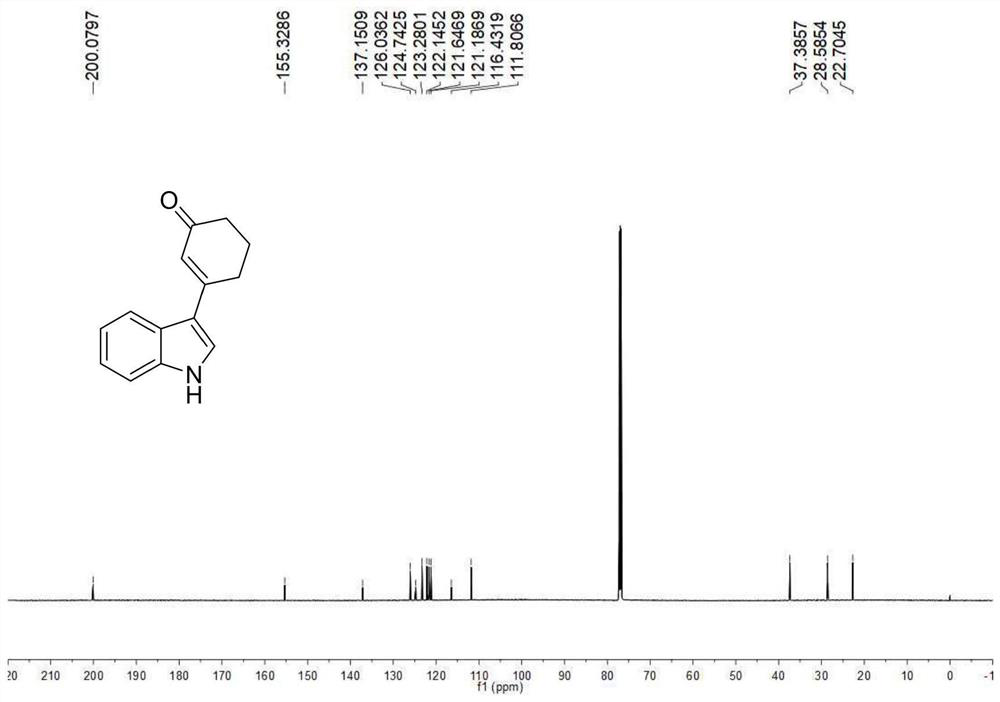
[0041] Example 1: Synthesis of 3-(1H-indol-3-yl)cyclohex-2-enone
[0042] (1) Indole (0.0293g, 0.25mmol), cyclohexenone (0.097mL, 1mmol), palladium trifluoroacetate (0.0083g, 0.025mmol), 2,5-dimethyl-8-trifluoromethane yl-3,4-dihydro-2H-pyrano[2,3-b]quinoline (0.0141 g, 0.05 mmol), tert-butanol peroxide (0.075 mL, 0.375 mmol), dimethyl sulfoxide (0.8 mL), tetrahydrofuran (0.4 mL), stirred evenly in a dry and clean airtight reaction tube, heated to 50° C., and reacted for 24 hours.
[0043](2) After the reaction was completed, the reaction tube was cooled to room temperature, 50 mL of ethyl acetate was added to dilute the reaction solution and transferred to a 100 mL separatory funnel, 10 mL of saturated brine was added, shaken, and allowed to stand, and the organic phase and the aqueous phase were separated, The aqueous phase was then extracted twice with 30 mL of ethyl acetate, the resulting organic phases were combined and dried over anhydrous sodium sulfate, the solvent wa...
Embodiment 2
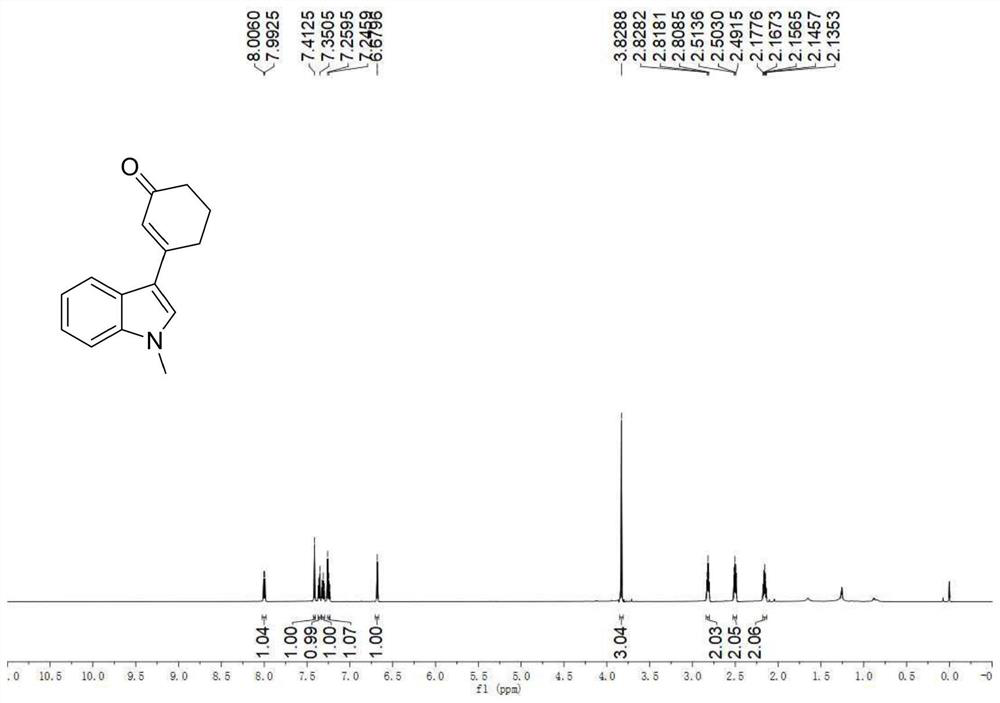
[0044] Example 2: Synthesis of 3-(1-methyl-indol-3-yl)cyclohex-2-enone
[0045] (1) 1-methylindole (0.031 mL, 0.25 mmol), cyclohexenone (0.097 mL, 1 mmol), palladium trifluoroacetate (0.0083 g, 0.025 mmol), 2,5-dimethyl-8 - trifluoromethyl-3,4-dihydro-2H-pyrano[2,3-b]quinoline (0.0141 g, 0.05 mmol), tert-butanol peroxide (0.075 mL, 0.375 mmol), dimethyl Sulfoxide (0.8 mL) and tetrahydrofuran (0.4 mL) were stirred uniformly in a dry and clean closed reaction tube, and then heated to 50° C. and reacted for 24 hours.
[0046] (2) After the reaction was completed, the reaction tube was cooled to room temperature, 50 mL of ethyl acetate was added to dilute the reaction solution and transferred to a 100 mL separatory funnel, 10 mL of saturated brine was added, shaken, and allowed to stand, and the organic phase and the aqueous phase were separated, The aqueous phase was then extracted twice with 30 mL of ethyl acetate, the resulting organic phases were combined and dried over anhyd...
Embodiment 3
[0047] Example 3: Synthesis of 3-(7-Methoxy-1H-indol-3-yl)cyclohex-2-enone
[0048] (1) 7-methoxyindole (0.033 mL, 0.25 mmol), cyclohexenone (0.097 mL, 1 mmol), palladium trifluoroacetate (0.0083 g, 0.025 mmol), 2,5-dimethyl -8-Trifluoromethyl-3,4-dihydro-2H-pyrano[2,3-b]quinoline (0.0141 g, 0.05 mmol), tert-butanol peroxide (0.075 mL, 0.375 mmol), bis Methyl sulfoxide (0.8 mL) and tetrahydrofuran (0.4 mL) were stirred uniformly in a dry and clean closed reaction tube, and then heated to 50° C. to react for 24 hours.
[0049] (2) After the reaction was completed, the reaction tube was cooled to room temperature, 50 mL of ethyl acetate was added to dilute the reaction solution and transferred to a 100 mL separatory funnel, 10 mL of saturated brine was added, shaken, and allowed to stand, and the organic phase and the aqueous phase were separated, The aqueous phase was then extracted twice with 30 mL of ethyl acetate, the resulting organic phases were combined and dried over an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com