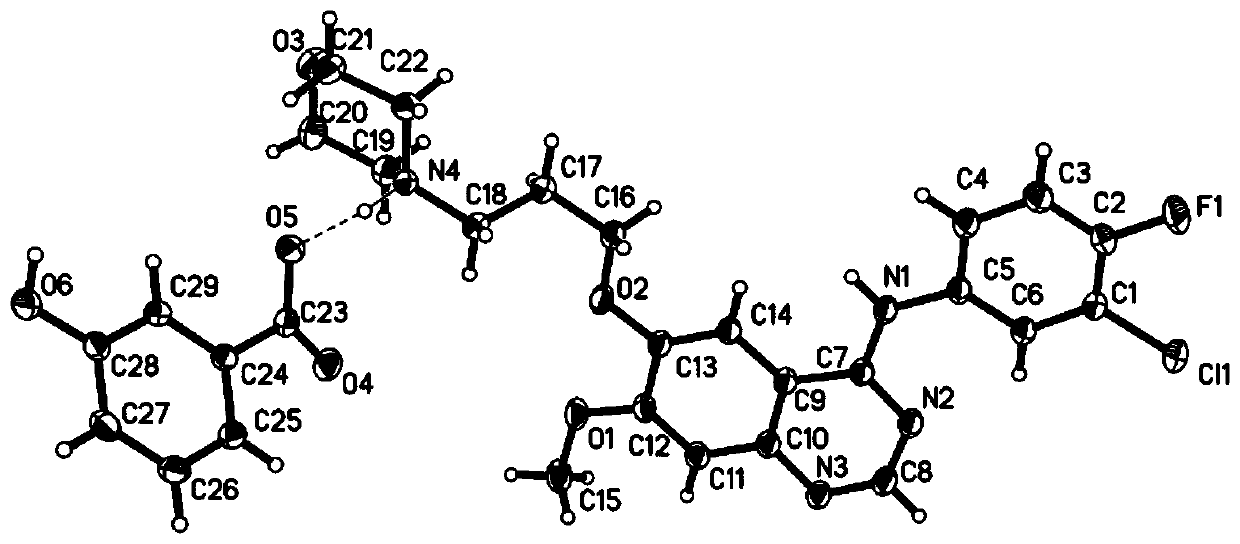
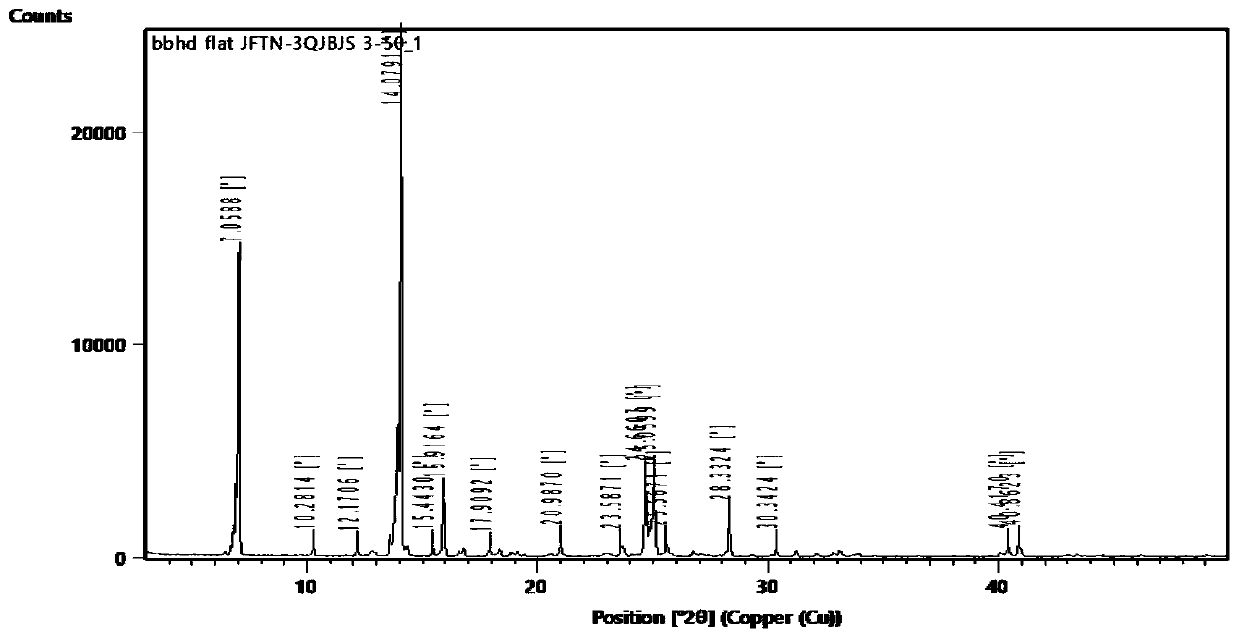
Gefitinib and 3-hydroxybenzoic acid eutectic
A technology of hydroxybenzoic acid and gefitinib, which is applied in the field of gefitinib and 3-hydroxybenzoic acid co-crystal and its preparation, can solve the problems of excessive organic solvent residue, low solubility, poor reproducibility, etc., and achieve Inhibition of the formation and crystallization phenomenon, good reproducibility, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 45.0mg of gefitinib and 11.6mg of 3-hydroxybenzoic acid into the mortar, and dropwise add 0.5mL of methanol, fully grind for 35min, then add 1mL of methanol and continue grinding for 15min to obtain a transparent solution. Stand at 5-10°C for crystallization for 48 hours, filter, and vacuum-dry at 55°C for 8 hours to obtain gefitinib-3-hydroxybenzoic acid co-crystal with a yield of 91.12%, HPLC: 99.94%.
Embodiment 2
[0054] Add 35.0mg of gefitinib and 11.0mg of 3-hydroxybenzoic acid into the mortar, and dropwise add 0.5mL of ethanol to it, thoroughly grind for 30min, then add 1mL of ethanol and continue grinding for 10min to obtain a transparent solution, control the temperature Stand at 5-10°C for crystallization for 48 hours, filter, and vacuum-dry at 55°C for 10 hours to obtain gefitinib-3-hydroxybenzoic acid co-crystal with a yield of 90.52%, HPLC: 99.92%.
Embodiment 3
[0056] Add 45.0mg of gefitinib and 11.6mg of 3-hydroxybenzoic acid into the mortar, and dropwise add 0.5mL of methanol, fully grind for 50min, then add 1mL of methanol and continue grinding for 20min to obtain a transparent solution. Stand at 5-10°C for crystallization for 48 hours, filter, and vacuum-dry at 55°C for 8 hours to obtain gefitinib-3-hydroxybenzoic acid co-crystal with a yield of 90.76%, HPLC: 99.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com