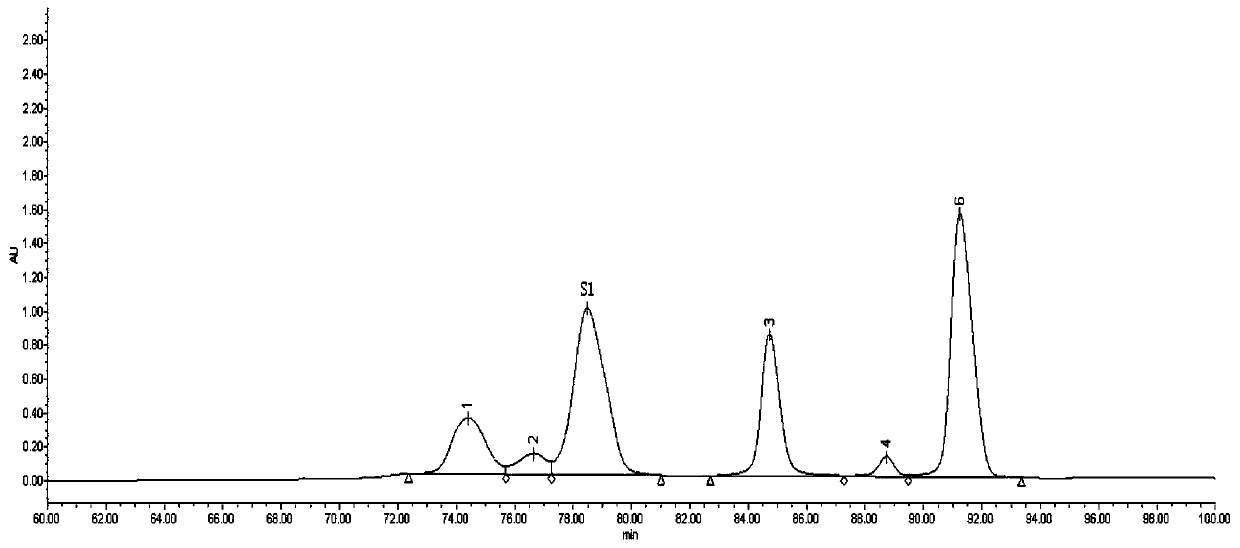
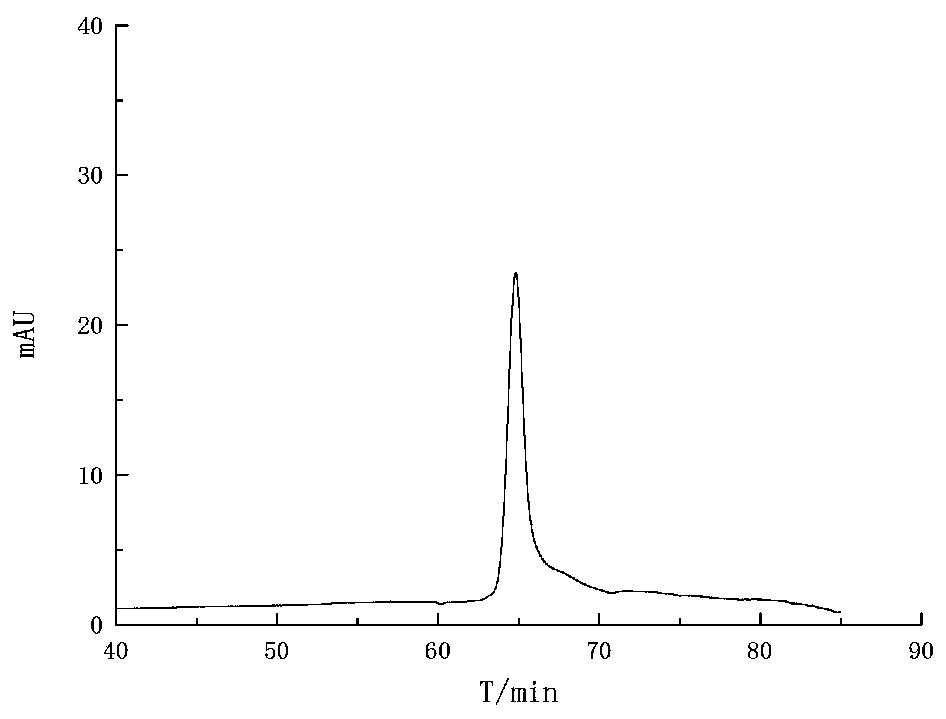
Steady-state malvidin 3-O-glucoside derivative and preparation method and applications thereof
A technology of glycoside derivatives and glucoside, which is applied in the field of stable mallow 3-O-glucoside derivatives and its preparation, can solve problems such as inability to meet production needs, low efficiency, and no reports, and achieve good light Effects of thermostability and anti-oxidative stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Wild grape pretreatment: After the wild grape is screened, removed, and cleaned, use a colloid mill at 2000r / min to grind for 20min to obtain a wild grape slurry for subsequent use;
[0044] (2) Extraction: 0.1% hydrochloric acid-75% ethanol (v / v) according to the liquid-to-material ratio of 5:1 (w / w), that is, 100mL solution contains 0.1mL of HCl and 75mL of ethanol, and the rest is deionized water ) was added to the grape grinding slurry, extracted by high-voltage pulsed electric field, the electric field strength was 10kV / cm, and the number of pulses was 10, to obtain anthocyanin extract;
[0045] (3) Centrifugal concentration: centrifuge the above anthocyanin extract at 3000r / min for 15min, discard the filter residue, take the supernatant, concentrate under reduced pressure at 45°C, 40r / min, and vacuum 0.08Mpa to a density of 1.0× 10 3 kg / m 3 , to obtain anthocyanin concentrate;
[0046] (4) Purification of D101 macroporous resin: dilute the concentrated anth...
Embodiment 2
[0053] (1) Wild grape pretreatment: After the wild grape is screened, removed, and cleaned, use a colloid mill at 4000r / min to grind for 10 minutes to obtain the wild grape slurry, which is set aside;
[0054] (2) Extraction: 0.5% hydrochloric acid-55% ethanol (v / v, i.e. 100mL solution contains 0.5mL of HCl and 55mL of ethanol, the rest being deionized water) extract was added to the grape grinding slurry, and extracted by high-voltage pulsed electric field, the electric field strength was 20kV / cm, and the number of pulses was 4 to obtain anthocyanin extract;
[0055] (3) Centrifugal concentration: centrifuge the above anthocyanin extract at 5000r / min for 5min, discard the filter residue, take the supernatant, concentrate under reduced pressure at 55°C, 60r / min, and vacuum 0.1Mpa to a density of 1.1× 10 3 kg / m 3 , to obtain anthocyanin concentrate;
[0056] (4) Purification of D101 macroporous resin: Dilute the concentrated anthocyanin solution 10 times with distilled water...
Embodiment 3
[0063] (1) Wild grape pretreatment: After the wild grape is screened, removed, and cleaned, use a colloid mill 3500r / min to grind for 15 minutes to obtain the wild grape slurry, which is set aside;
[0064] (2) Extraction: 0.1% hydrochloric acid-65% ethanol (v / v, i.e. 100mL solution contains 0.1mL of HCl and 65mL of ethanol, the rest being deionized water) extract was added to the grape grinding slurry, and extracted by high-voltage pulsed electric field, the electric field strength was 15kV / cm, and the number of pulses was 6 to obtain anthocyanin extract;
[0065](3) Centrifugal concentration: centrifuge the above anthocyanin extract at 4500r / min for 10min, discard the filter residue, take the supernatant, concentrate under reduced pressure at 50°C, 50r / min, and vacuum 0.09Mpa to a density of 1.05× 10 3 kg / m 3 , to obtain anthocyanin concentrate;
[0066] (4) Purification of D101 macroporous resin: dilute the anthocyanin concentrate 5 times with deionized water, and load t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com