Lactobacillus gasseri for resisting enterohemorrhagic escherichia coli, application of lactobacillus gasseri, composite probiotic preparation for poultry, prepared from lactobacillus gasseri and application of composite probiotic preparation
A technology of Lactobacillus gasseri and compound probiotics, applied in the direction of bacteria, Lactobacillus, and other applications used in food preparation, can solve the problems of poor vaccine control effects, drug residues, drug resistance, etc., and improve intestinal Mucosal immune level, improvement of resistance, effect of enhancing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
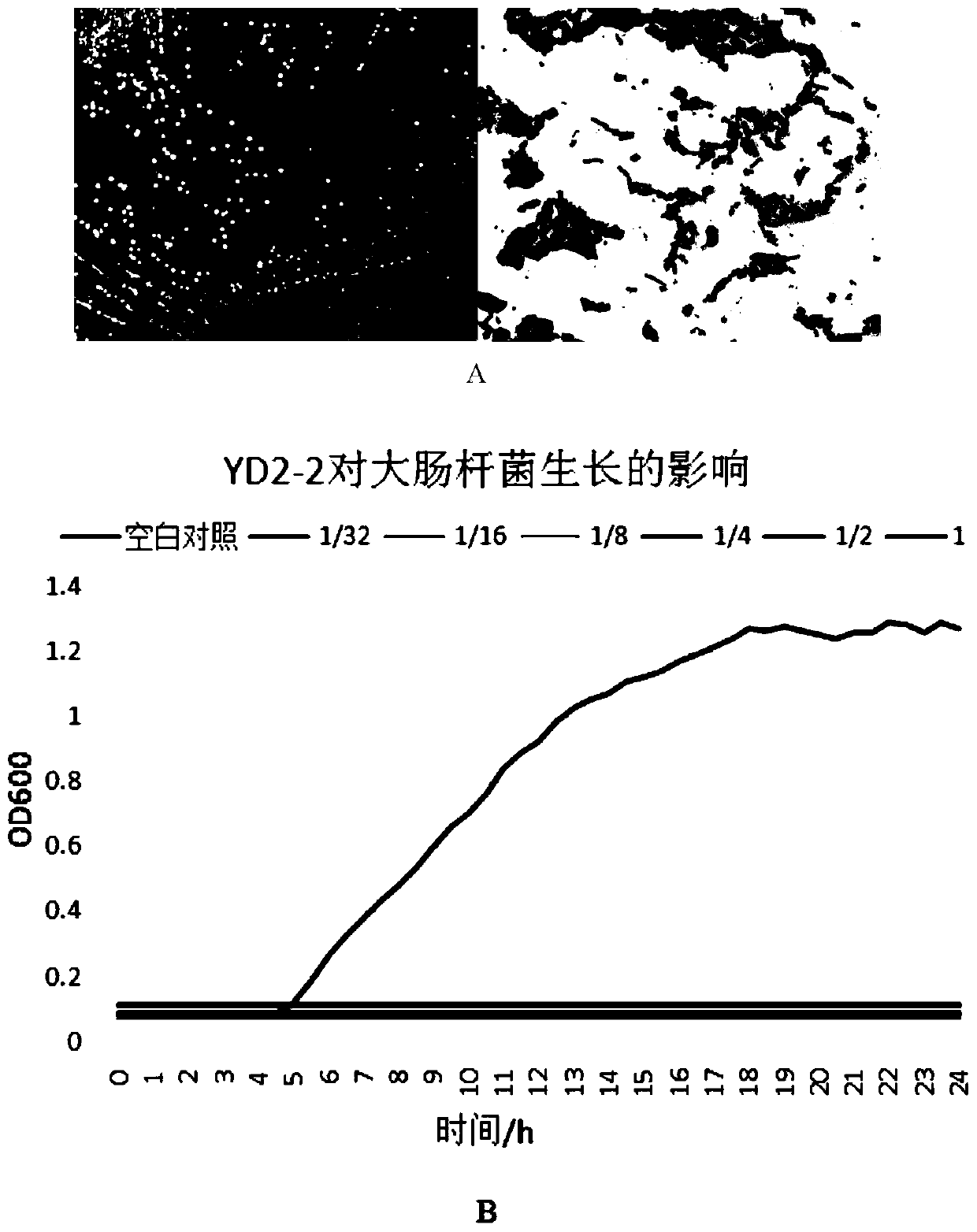
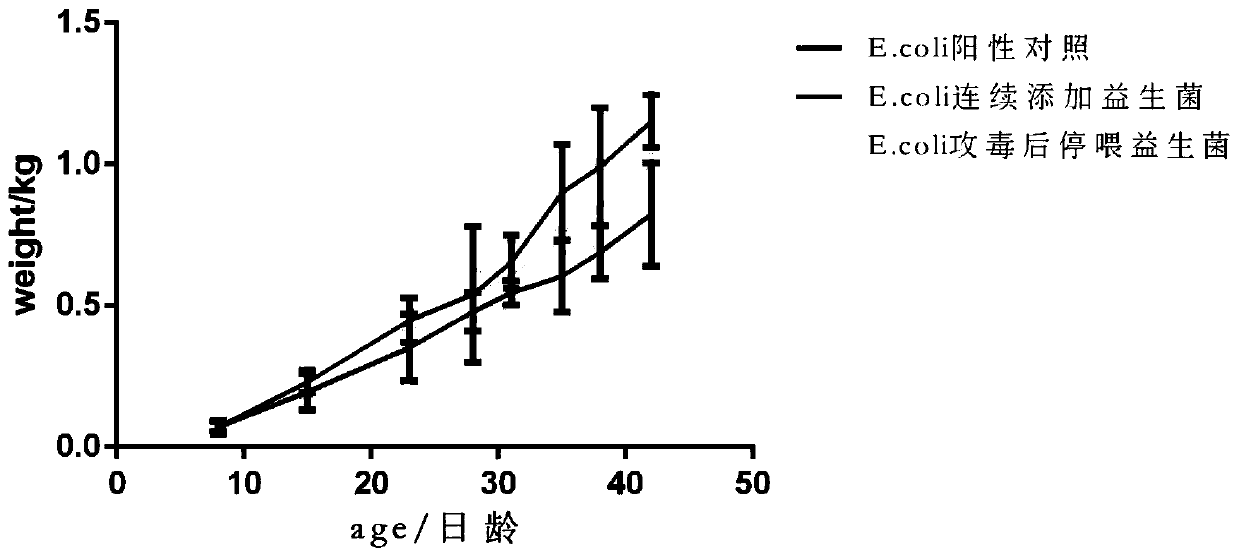
Image
Examples
Embodiment 1
[0033] Isolation and identification of Lactobacillus gasseri YD2-2:
[0034] 1) Purchase healthy free-range chickens from a chicken farm in Xianning, dissect the intestines, and take the contents of the intestines in a sterile environment. After shaking and mixing, they are diluted in multiples, and the appropriate dilution is selected and spread on the lactic acid bacteria selective medium MRS agar culture. On the substrate, culture anaerobically at 37°C for 24-48h, pick a single colony on the plate, streak and purify it on the MRS medium plate several times, and cultivate it aerobically at 37°C to obtain pure colonies. Colony morphology observation and Gram staining were performed on the purified strains, and Gram-positive bacilli were selected. The colony diameter of the bacterial strain of the present invention is 0.5-1mm on the MRS medium, milky white, raised, round in shape, smooth in surface and facultative anaerobic. The strain is Gram-positive, round-ended straight r...
Embodiment 2
[0043] Effect of Lactobacillus gasseri YD2-2 on immune cytokines in mice:
[0044] Select 4-week-old female Kunming rats as experimental animals, and use the method of gavage for 1 week, once a day, each time with 0.2ml of YD2-2 fermentation broth, the amount of bacteria contained in the fermentation broth is 10 8 ~10 9 cfu / ml. Normal feeding for one week before gavage, and normal feeding for one week after gavage. The whole experiment was 21 days. After the experiment, eyeball blood was collected to measure cytokines, and the effect of YD2-2 on mouse IFNβ and IL-1β cytokines was determined by fluorescent quantitative PCR, and GAPDH was used as an internal reference gene. The results of fluorescence quantitative detection of IFNβ and IL-1β are as follows:
[0045] Table 2 Effect of Lactobacillus gasseri YD2-2 on immune cytokines in mice.
[0046]
[0047] Assume that the amplification efficiencies of both the target gene and the reference gene are close to 100%, and the...
Embodiment 3
[0053] A compound probiotic preparation for anti-enteritis Escherichia coli infection, including Bacillus subtilis, Bacillus coagulans, Bacillus amyloliquefaciens, Lactobacillus gasseri and Lactobacillus plantarum; the number of viable bacteria of Bacillus subtilis is 10 9 CFU / g, the number of viable Bacillus coagulans is 10 8 CFU / g, the number of live bacteria of Bacillus amyloliquefaciens is 10 8 CFU / g, the viable count of Lactobacillus gasseri is 10 8 CFU / g, the number of viable Lactobacillus plantarum is 10 8 CFU / g.
[0054] The Bacillus amyloliquefaciens is Bacillus amyloliquefaciens H6, CCTCC NO: M2013249; the Lactobacillus plantarum is Lactobacillus plantarum ZN-3, CCTCC NO: M 2017286, and the Lactobacillus gasseri is Lactobacillus gasseri YD2-2, CCTCC NO: M2019340, other strains were purchased from commercial channels.
[0055] The composite probiotic preparation prepared in Example 3 is used in the following examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com