Preparation method and application of cytarabine-modified methotrexate-loaded liposome
A technology of carrying methotrexate lipids and carrying methotrexate, which is applied in the field of biomedicine and can solve problems such as serious side effects, high disease recurrence rate, and low specificity of methotrexate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
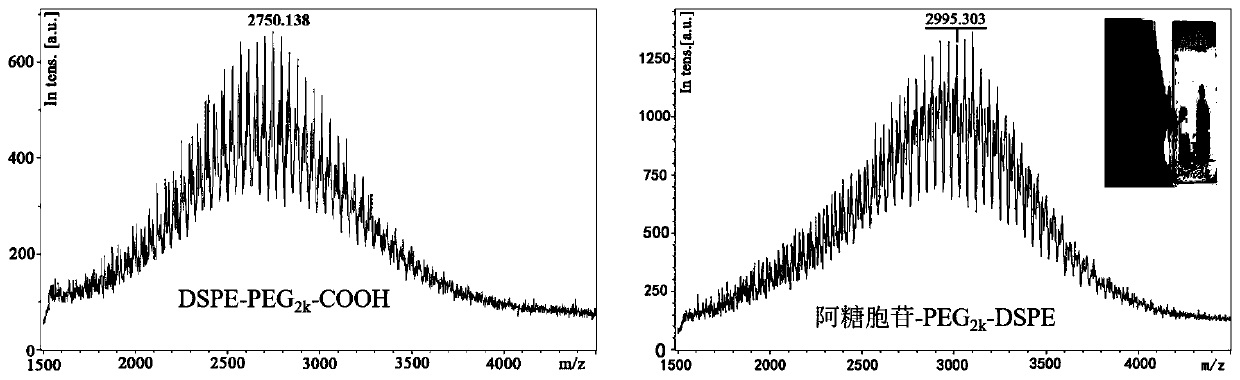
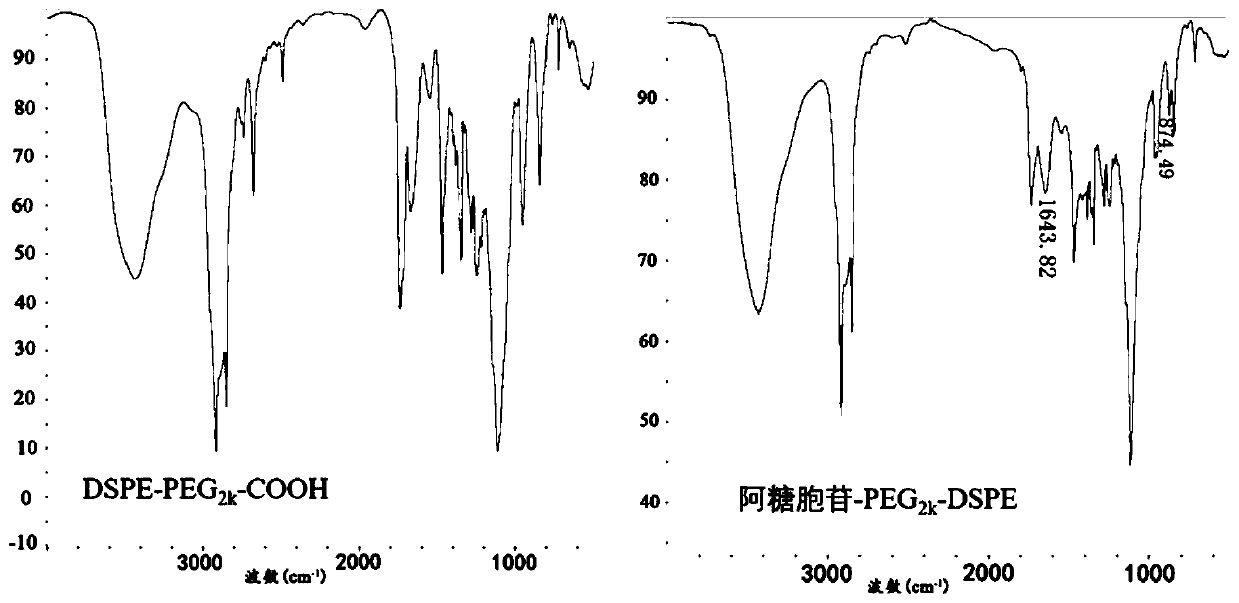
[0029] 1. Cytarabine-PEG 2k -Synthesis of DSPE:
[0030] (1) Accurately weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolved in 10ml of dimethylformamide and shaken well. Then accurately weigh 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt), were sequentially added to the above-mentioned dimethylformamide solution system, stirred in an ice bath for 3 hours, and DSPE-PEG 2k - The molar ratio of COOH, cytarabine, HATU, HOBt to triethylamine is 1:1.2:1.2:1.2:2.
[0031] (2) After the reaction in (1) is completed, move the reaction system to a dialysis bag with a molecular weight cut-off of 3500 Da, dialyze in 1000 ml deionized water for 72 hours, and change the deionized water every 6 hours.
[0032] (3) After the dialysis, the liquid in the dialysis bag was cooled and freeze-dried for 48 hours to obtain cytarabine-PEG 2k -DSPE.
[0033] 2. Prepara...
Embodiment 2
[0043] 1. Cytarabine-PEG 2k -Synthesis of DSPE:
[0044] (1) Accurately weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolved in 10ml of dimethylformamide and shaken well. Then accurately weigh 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt), were sequentially added to the above-mentioned dimethylformamide solution system, stirred in an ice bath for 3 hours, and DSPE-PEG 2k - The molar ratio of COOH, cytarabine, HATU, HOBt to triethylamine is 1:2:1.2:1.2:2.
[0045] (2) After the reaction in (1) is completed, move the reaction system to a dialysis bag with a molecular weight cut-off of 3500 Da, dialyze in 1000 ml deionized water for 72 hours, and change the deionized water every 6 hours.
[0046] (3) After the dialysis, the liquid in the dialysis bag was cooled and freeze-dried for 48 hours to obtain cytarabine-PEG 2k -DSPE.
[0047] 2. Preparati...
Embodiment 3
[0055] 1. Cytarabine-PEG 2k -Synthesis of DSPE:
[0056] (1) Accurately weigh 140.00mg DSPE-PEG 2k -COOH (MW: 2800), dissolved in 10ml of dimethylformamide and shaken well. Then accurately weigh 14.60mg cytarabine (MW: 243.22), 22.80mg 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate ( HATU), triethylamine, and 1-hydroxybenzotriazole (HOBt), were sequentially added to the above-mentioned dimethylformamide solution system, stirred in an ice bath for 5h, wherein DSPE-PEG 2k - The molar ratio of COOH, cytarabine, HATU, HOBt to triethylamine is 1:1.2:1.2:1.2:2.
[0057] (2) After the reaction in (1) is completed, move the reaction system to a dialysis bag with a molecular weight cut-off of 3500 Da, dialyze in 1000 ml deionized water for 72 hours, and change the deionized water every 6 hours.
[0058] (3) After the dialysis, the liquid in the dialysis bag was cooled and freeze-dried for 48 hours to obtain cytarabine-PEG 2k -DSPE.
[0059] 2. Preparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com