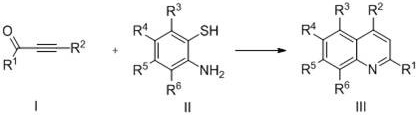
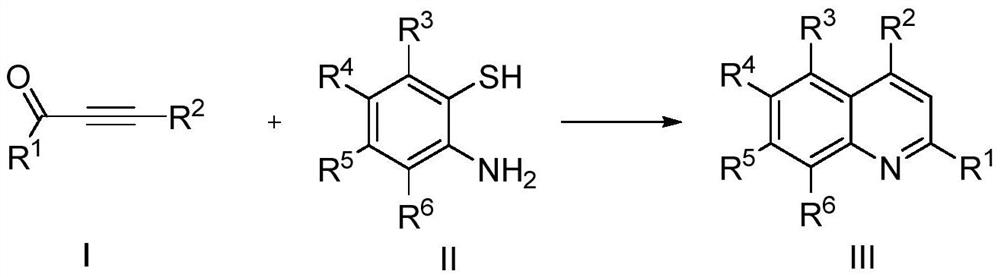
Method for preparing quinoline compounds by catalysis of zirconocene dichloride
A zirconocene dichloride, catalytic preparation technology, applied in the direction of organic chemistry, etc., to achieve the effect of mild reaction conditions, simple operation, and stable air
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
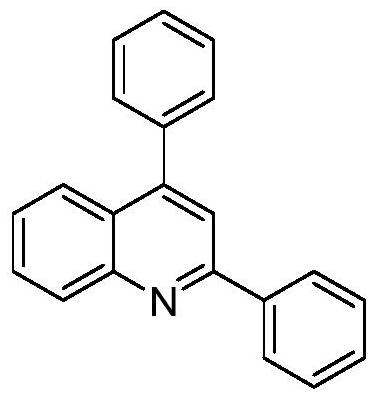
Embodiment 1
[0017] Preparation of 2,4-diphenylquinoline with the following structural formula
[0018]
[0019] Add 0.103g (0.5mmol) 1,4-diphenyl-3-butyne-2-one, 0.0073g (0.025mmol) zirconocene dichloride, 0.0082g (0.05mmol) L-phenylpropane to the reaction flask Amino acid, 64μL (0.6mmol) o-aminobenzenethiol, 1mL DMF, stirred and reacted at 30°C for 5 hours, then added 0.1518g (0.6mmol) iodine, continued to stir and reacted at 30°C for 2 hours, stopped the reaction, added Add 10 mL of saturated sodium thiosulfate aqueous solution, add 10 mL of ethyl acetate and extract 3 times, remove the ethyl acetate by rotary evaporation of the organic phase, and separate with a silica gel column (the eluent is petroleum ether and dichloromethane in a volume ratio of 2:1) Mixed liquor), obtains 2,4-diphenylquinoline, and its productive rate is 93%, and the spectral data of product is: 1 H NMR (400MHz, CDCl 3 )δ8.16(d, J=8.4Hz, 1H), 8.10(d, J=7.4Hz, 2H), 7.81(d, J=8.3Hz, 1H), 7.72(s, 1H), 7.63(t, ...
Embodiment 2
[0025] Preparation of 2-(4-chlorophenyl)-4-phenylquinoline with the following structural formula
[0026]
[0027] In this example, the 1,4-diphenyl-3-butane used in Example 1 was replaced with equimolar 1-(4-chlorophenyl)-4-phenyl-3-butyn-2-one Alkyn-2-ketone, other steps are identical with embodiment 1, obtain 2-(4-chlorophenyl)-4-phenylquinoline, and its productive rate is 84%, and the spectral data of product is: 1 H NMR (400MHz, CDCl 3 )δ8.12(d, J=8.4Hz, 1H), 8.05(d, J=8.4Hz, 2H), 7.80(d, J=8.3Hz, 1H), 7.68-7.60(m, 2H), 7.44( s,5H),7.38(d,J=8.2Hz,3H); 13 C NMR (101MHz, CDCl 3 )δ155.51, 149.41, 148.78, 138.27, 138.03, 135.58, 130.11, 129.70, 129.55, 129.01, 128.84, 128.65, 128.51, 126.55, 125.84, 125.69, 118.89.
Embodiment 3
[0029] Preparation of 2-(4-fluorophenyl)-4-phenylquinoline of the following structural formula
[0030]
[0031] In this example, the 1,4-diphenyl-3-butane used in Example 1 was replaced with equimolar 1-(4-fluorophenyl)-4-phenyl-3-butyn-2-one Alkyn-2-one, other steps are the same as in Example 1, obtain 2-(4-fluorophenyl)-4-phenylquinoline, its productive rate is 90%, and the spectral data of product is: 1 H NMR (400MHz, CDCl 3 )δ8.16-8.06 (m, 3H), 7.83-7.77 (m, 1H), 7.69-7.60 (m, 2H), 7.49-7.40 (m, 5H), 7.38 (ddd, J = 8.2, 6.8, 1.3 Hz,1H),7.11(t,J=8.7Hz,2H); 13 C NMR (101MHz, CDCl 3 )δ165.07,162.59,155.76,149.35,148.77,138.32,135.81,135.77,130.04,129.66,129.55,129.48,129.39,128.63,128.48,126.40,125.70,125.68,118.98,115.88,115.67.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com