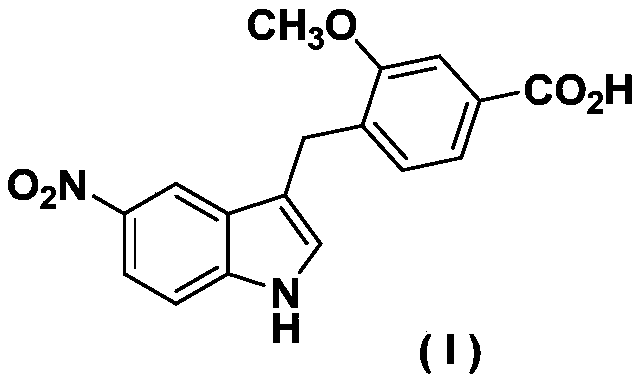
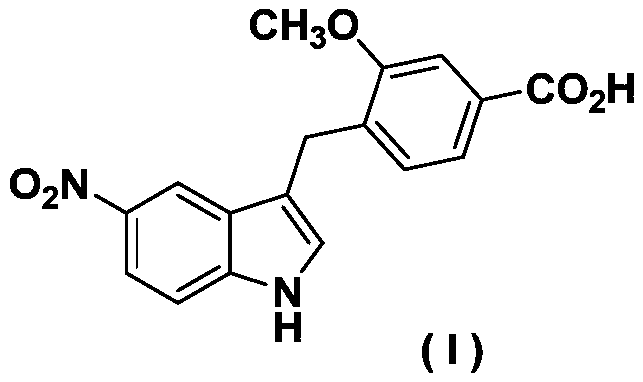
Zafirlukast intermediate preparation process
A preparation process and intermediate technology, which is applied in the field of preparation process of the asthma drug zafirlukast intermediate, can solve the problems of increased impurities, expensive raw materials/reagents, difficult purification, etc., and achieve high purity, low production cost, and post-processing Easy to handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of (5-nitro-1-toluenesulfonyl-1H-indol-3-yl)methyl acetate:
[0025]
[0026] In a 250mL round bottom flask, add 5-nitroindole-3-carbaldehyde (19g, 0.1mol) dissolved in 150mL of dry dichloromethane, then add p-toluenesulfonyl chloride (19.1g, 0.1mol) and carbonic acid Potassium (27.6g, 0.2mol) was heated under reflux for 12 hours. TLC determined that the raw material disappeared, cooled to room temperature, stood still, and filtered with suction, and the filtrate was distilled off under reduced pressure to remove the organic solvent to obtain 29 g of a yellow solid. In a 250 mL round bottom flask, 29 g of the above yellow solid was dissolved in 100 mL of ethanol, and sodium borohydride (6.4 g, 0.17 mol) was added in batches under stirring, and reacted at room temperature for 6 hours. TLC determined that the starting material disappeared, adjusted the pH to 7 with 1M hydrochloric acid aqueous solution, extracted with ethyl acetate (3×50 mL), combined the ...
Embodiment 2
[0036] Preparation of methyl 3-methoxy-4-[(5-nitro-1-tosyl-1H-indol-3-yl)methyl]benzoate:
[0037] In a 250 mL round bottom flask, methyl (5-nitro-1-tosyl-1H-indol-3-yl)acetate (9.7 g, 0.025 mol) and methyl 3-methoxybenzoate ( 4.2g, 0.025mol) was dissolved in 50mL of chloroform, acidified montmorillonite (8.4g) was added under stirring, and heated to 45°C for reflux reaction for 4 hours. TLC determined that the raw material disappeared, cooled to room temperature, stood still, suction filtered, and the filtrate was distilled off under reduced pressure to remove the organic solvent to obtain a yellow solid, which was recrystallized from ethyl acetate / cyclohexane to obtain a white solid 3-methoxy-4-[(5 -Methyl nitro-1-tosyl-1H-indol-3-yl)methyl]benzoate (8.1 g, 66% yield).
Embodiment 3
[0039] Preparation of methyl 3-methoxy-4-[(5-nitro-1-tosyl-1H-indol-3-yl)methyl]benzoate:
[0040] In a 250 mL round bottom flask, methyl (5-nitro-1-tosyl-1H-indol-3-yl)acetate (9.7 g, 0.025 mol) and methyl 3-methoxybenzoate ( 4.2 g, 0.025 mol) was dissolved in 50 mL of 1,2-dichloroethane, and acidified montmorillonite (8.4 g) was added under stirring, and heated to 45° C. for reflux reaction for 4 hours. TLC determined that the raw material disappeared, cooled to room temperature, left to stand, suction filtered, and the filtrate was distilled off under reduced pressure to remove the organic solvent to obtain a yellow solid, which was recrystallized from ethyl acetate / cyclohexane to obtain a white solid 3-methoxy-4-[(5 -Methyl nitro-1-tosyl-1H-indol-3-yl)methyl]benzoate (7.8 g, 63% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com