Cyclic peptide compound simulating structure of natural product and preparation method of cyclic peptide compound
A technology of natural products and compounds, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problem of amino acids not being able to be used, and achieve the effect of broadening the application range of hydrocarbon activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0069] Screening of reaction solvents:
[0070]
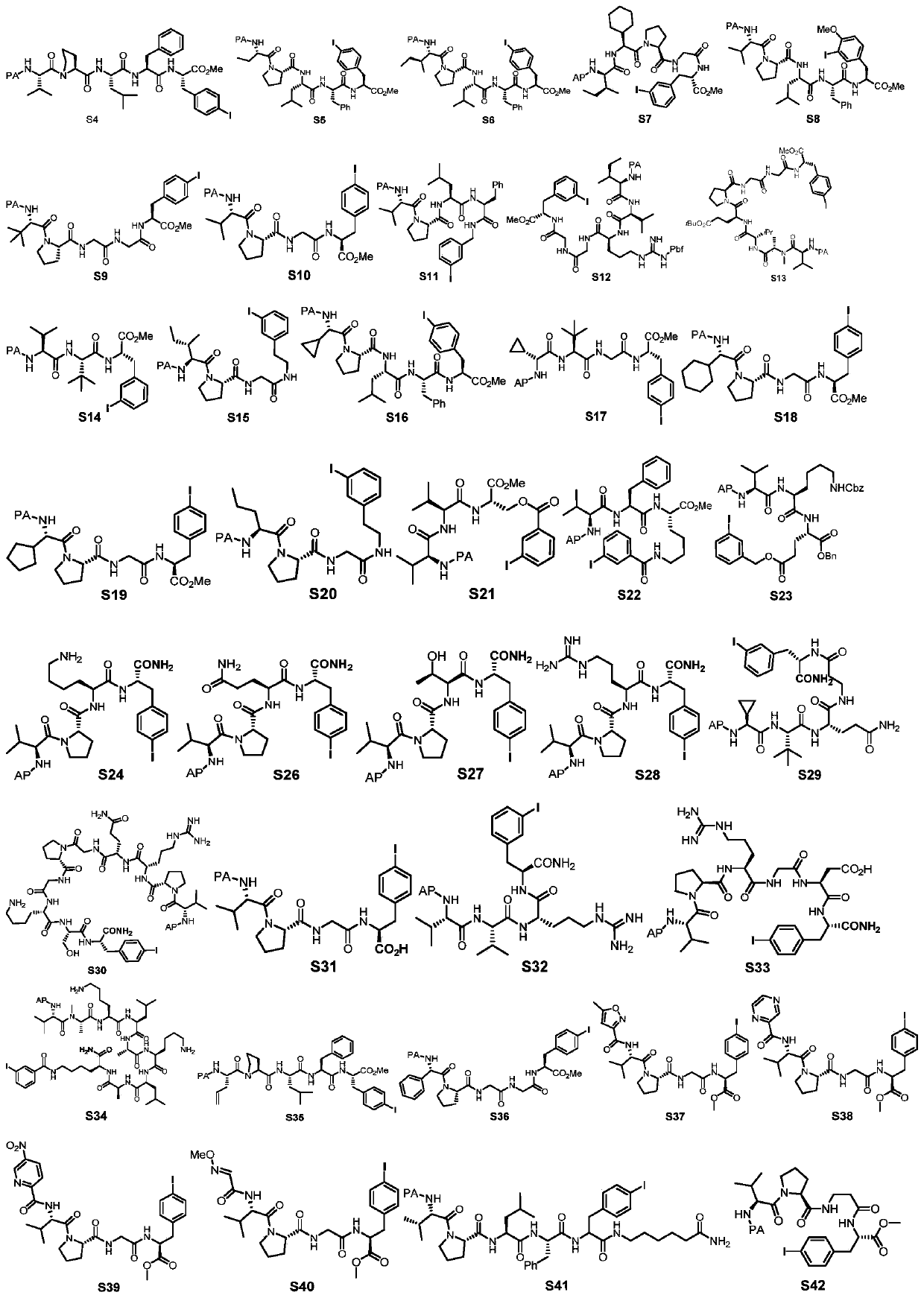
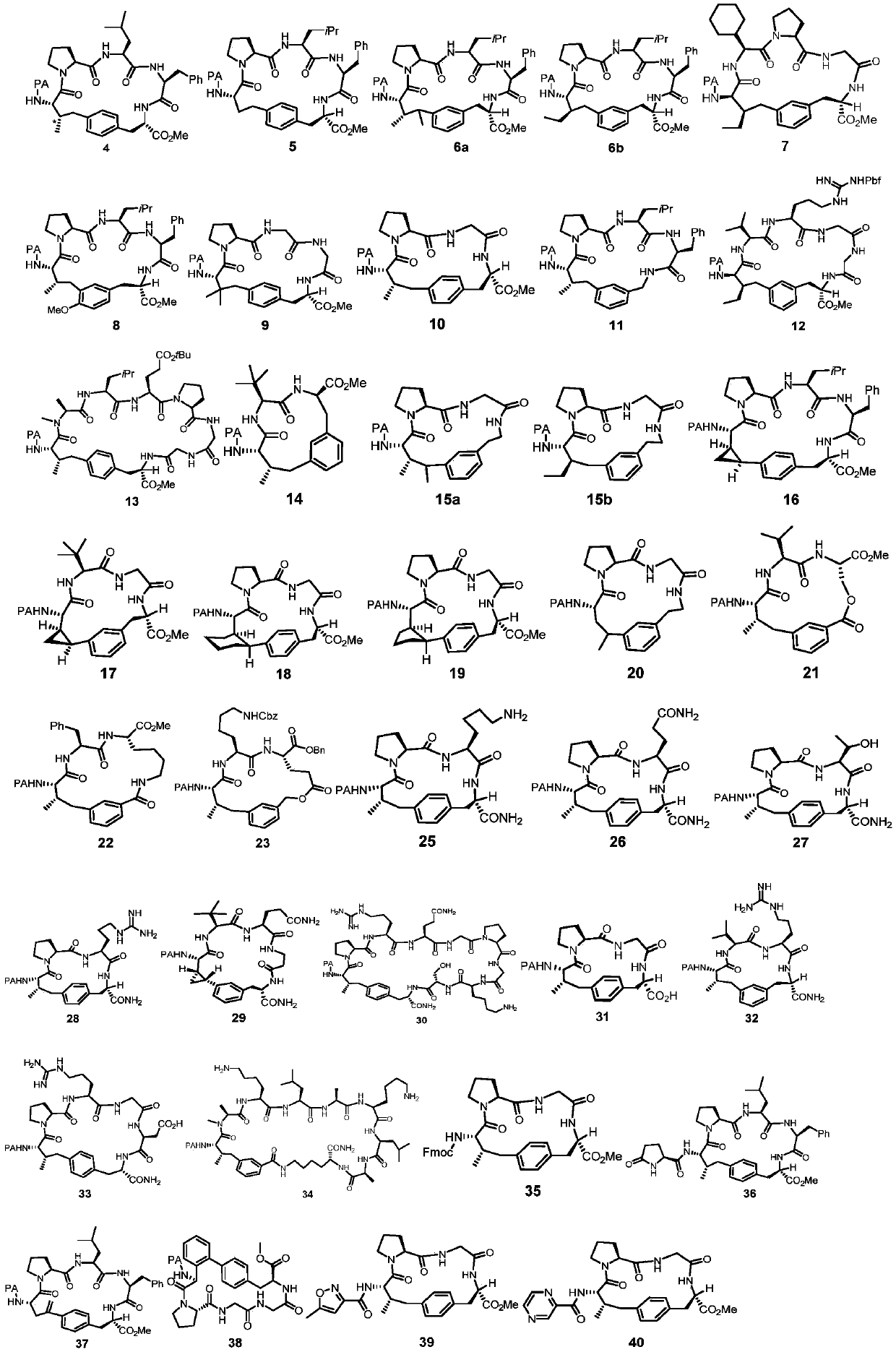
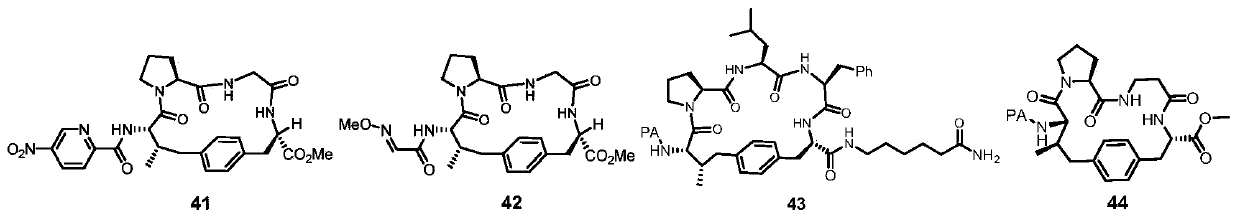
[0071]The linear polypeptide (No. S4) (43.4mg, 0.05mmol, 1.0equiv), AgOAc (12.6mg, 0.075mmol, 1.5equiv) and Pd (OAc) 2 (2.2mg, 10mol%) was weighed into an 8mL reaction vial (sealed with a PTFE lid), then 2mL of solvent was added at room temperature and stirred for 5 minutes, then heated to 110°C for 6 hours. The reaction was cooled to room temperature, 5 mL of acetone was added to the reaction system to dilute, and then filtered with celite, and the obtained filtrate was evaporated to dryness to obtain an oil, which was purified by column chromatography to obtain the final white ring-closing product. The difference between Examples 1-9 is only in the reaction solvent, as shown in Table 1.
[0072] The screening of table 1 reaction solvent
[0073]
[0074] As can be seen from the results of Examples 1-9, when the solvent is selected as HFIP, the yield is higher.
Embodiment 10-16
[0076] Screening of different divalent palladium metal catalysts:
[0077]
[0078] The preparation method of Examples 10-16 is basically the same as that of Example 6, except that the divalent palladium metal catalyst used in Examples 10-16 is different, as shown in Table 2.
[0079]
[0080] As can be seen from the results of Examples 10-16, when the palladium metal catalyst is selected as Pd(CH 3 EN) 4 (BF 4 ) 2 , the yield is higher.
Embodiment 17-24
[0082] Screening of different silver salts:
[0083]
[0084] The preparation method of Examples 17-24 is basically the same as that of Example 14, the only difference is that the silver salt used in Examples 17-24 is different, as shown in Table 3.
[0085] Table 3 Screening of different silver salts
[0086]
[0087] As can be seen from the results of Examples 17-24, when the silver salt is selected as the AgOAc of Example 14, the yield is higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com