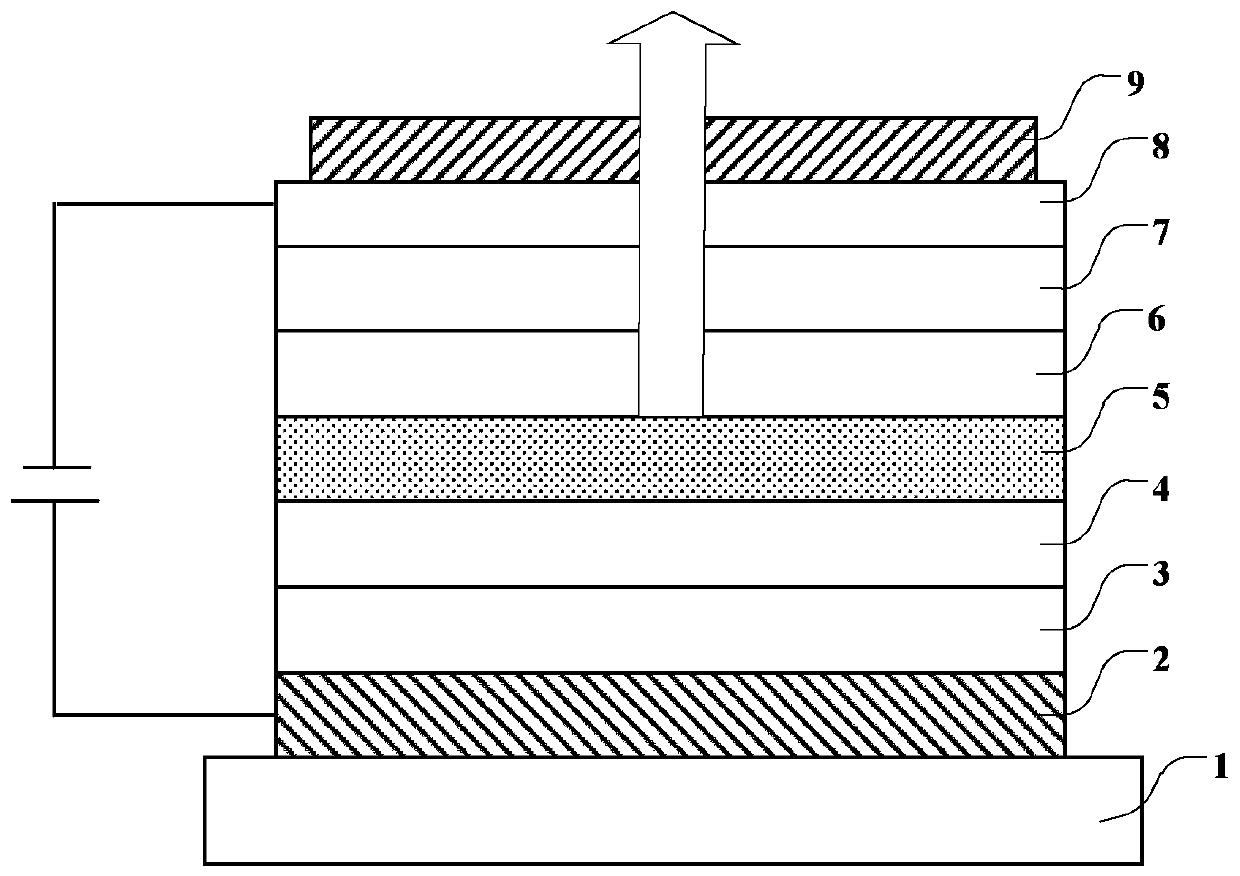
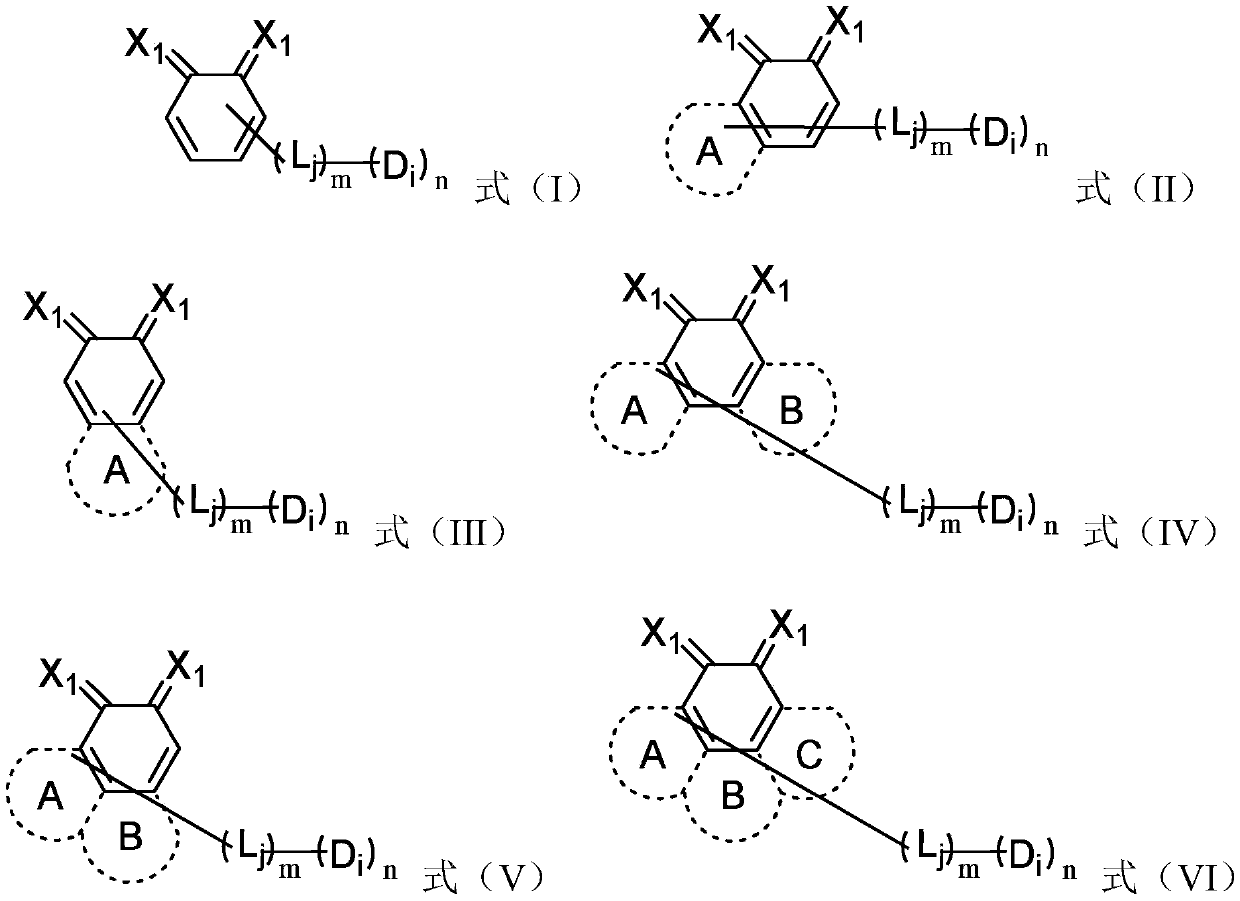
Compound, organic photoelectric device and electronic equipment
A technology of organic photoelectric devices and compounds, applied in the fields of compounds, organic photoelectric devices and electronic equipment, can solve the problems of device luminous efficiency and thermal stability to be further improved, and achieve high glass transition temperature and molecular thermal stability , high glass transition temperature, and the effect of reducing the driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0135] Compound P1 was prepared by the following method:
[0136]
[0137] Specific steps:
[0138] In a 250 mL round bottom flask, 9-(4-bromo-phenyl)-10-(4-iodo-phenyl)-anthracene (15 mmol), diphenylarylamine (15 mmol), cuprous oxide (40 mmol), DMAC (20mL), refluxed under argon atmosphere for 48 hours, the resulting intermediate was cooled to room temperature, added to water, and then filtered through a celite pad, the filtrate was extracted with dichloromethane, then washed with water, and dried over anhydrous magnesium sulfate , after filtration and evaporation, the crude product was purified by silica gel column chromatography to obtain intermediate product P1-1;
[0139] In a 250 mL round bottom flask, intermediate P1-1 (15 mmol) and potassium acetate (40 mmol) were mixed with dry 1,4-dioxane (60 mL), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting intermediate wa...
preparation example 2
[0143] Compound P16 was prepared by the following method:
[0144]
[0145] Specific steps:
[0146]In a 250 mL round bottom flask, 9-(4-bromo-phenyl)-10-(4-iodo-phenyl)-anthracene (15 mmol), 9-H carbazole (15 mmol), cuprous oxide (40 mmol) , DMAC (20mL), refluxed under argon atmosphere for 48 hours, the obtained intermediate was cooled to room temperature, added to water, then filtered through a celite pad, the filtrate was extracted with dichloromethane, then washed with water, and treated with anhydrous sulfuric acid After drying over magnesium, filtration and evaporation, the crude product was purified by silica gel column chromatography to give intermediate product P16-1;
[0147] In a 250 mL round bottom flask, intermediate P16-1 (15 mmol) and potassium acetate (40 mmol) were mixed with dry 1,4-dioxane (60 mL), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting inte...
preparation example 3
[0151] Compound P32 was prepared by the following method:
[0152]
[0153] Specific steps:
[0154] In a 250 mL round bottom flask, 5-chloro-2-iodo-pyrimidine (15 mmol), 9-H carbazole (15 mmol), cuprous oxide (40 mmol), DMAC (20 mL) were refluxed under argon atmosphere for 48 hours , the resulting intermediate was cooled to room temperature, added to water, then filtered through a pad of celite, the filtrate was extracted with dichloromethane, then washed with water, and dried with anhydrous magnesium sulfate, filtered and evaporated, purified by silica gel column chromatography The crude product gives the intermediate product P32-1;
[0155] In a 250 mL round bottom flask, intermediate P32-1 (15 mmol) and potassium acetate (40 mmol) were mixed with dry 1,4-dioxane (60 mL), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting intermediate was cooled to room temperature, ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com