Polypeptide for inhibiting actin recombination and application thereof
A technology of actin and preparation, which is applied in the field of peptides and applications that inhibit actin recombination, and achieves the effect of weak immunogenicity and strong specific binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
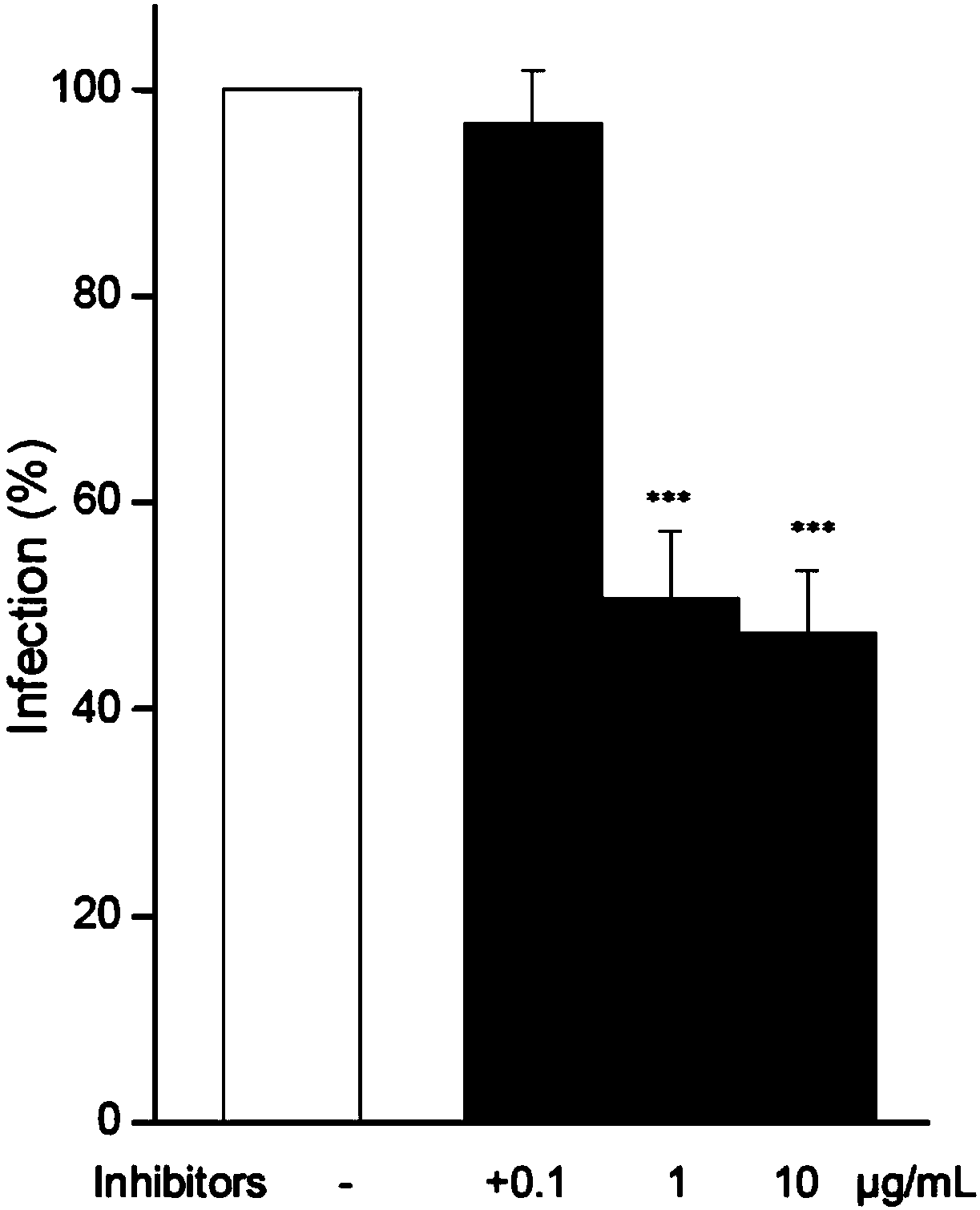
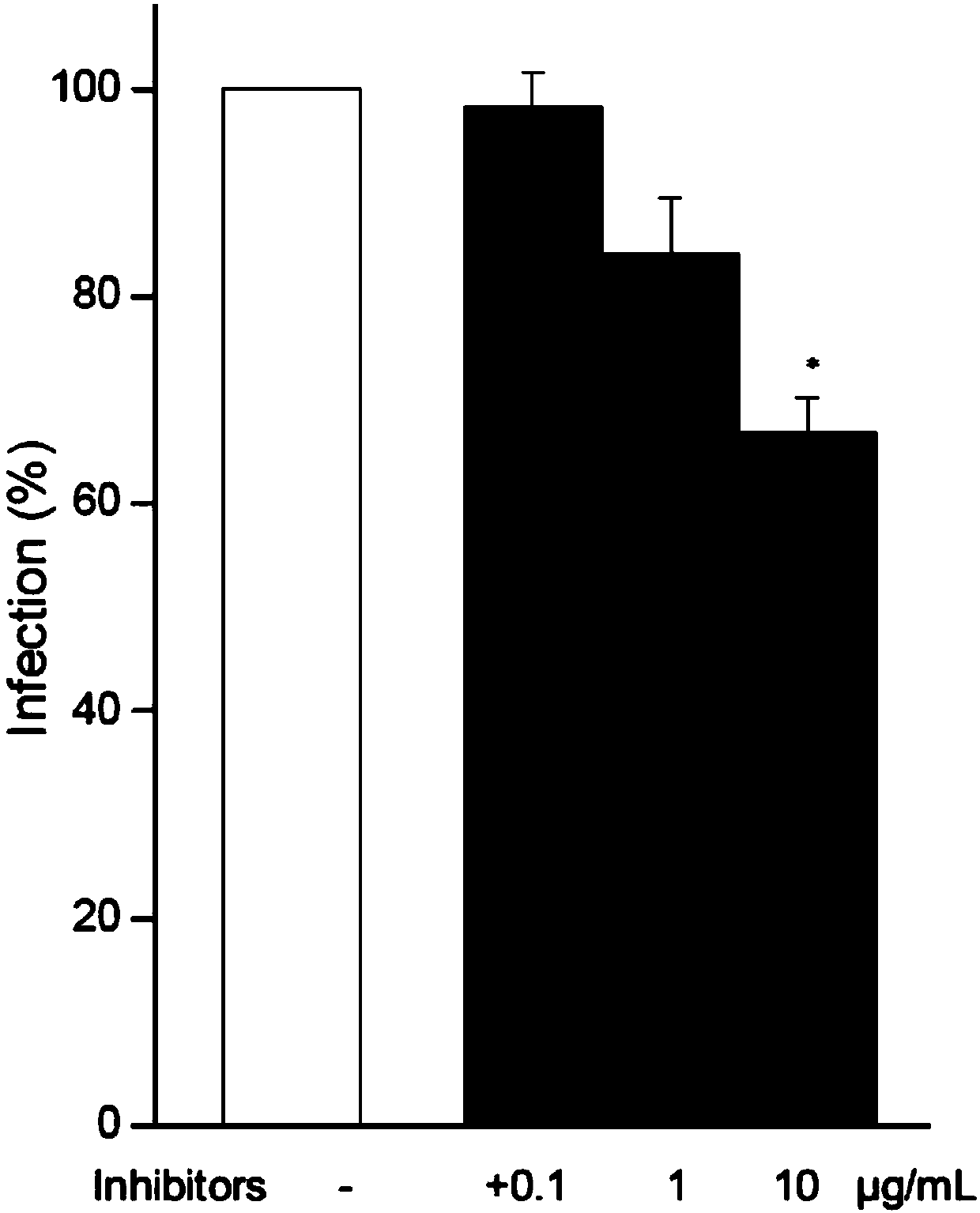
[0028] HIV-1 entry into CD4 by ABS1p + Inhibitory effect of T cells or CEM-ss cells
[0029] Studying the effect of ABS1p on HIV-1 entry into CD4 + For the inhibitory effect of T cells or CEM-ss cells, first adjust the cell concentration to 1×10 6 cells / mL, the volume of each group of cells is 2mL; add peptide ABS1p to the cell system, and incubate in a 37°C incubator for 1h; after pretreatment, add 200ng p24HIV-1 to the cells, place at 37°C, 5% CO 2 After the infection was completed, the cells were rinsed with PBS for 3 times, and fresh medium was added to make the cell concentration 1×10 6 cells / mL, keep at 37°C, 5% CO 2 Conditioned for 48h.
[0030]When measuring the infection effect of the virus on the cells, Intracellular p24staining was used to detect the p24 content in the cells after virus infection. In the experiment, the virus-infected cells were first collected, washed with PBS for 3 times, and then p24 in the cells were stained according to the Anti-HIV-1p24an...
Embodiment 2
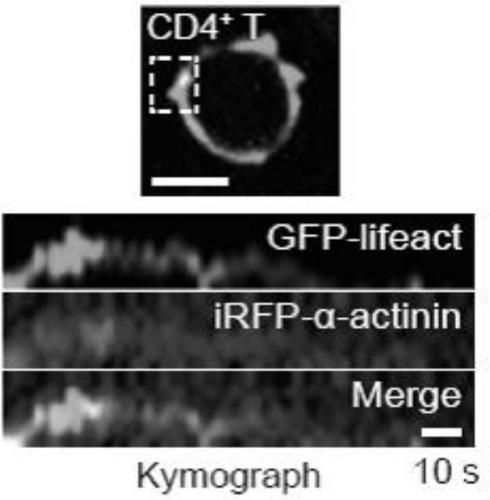
[0033] ABS1p affects the recombination of F-actin and α-actinin in T cells:
[0034] order in CD4 + To study the dynamic relationship between F-actin and α-actinin in T cells and T cell line CEM-ss cells, co-transfect CD4 with plasmid pECFP-C1-lifeact and piRFP-α-actinin + T cells or CEM-ss cells can achieve fluorescent labeling of F-actin and α-actinin in living cells. The specific implementation process is: (1) add 100 μ L room temperature Solution resuspended CD4 + Add 2 μg pECFP-C1-Lifeact and 2 μg piRFP-α-actinin to T cells or CEM-ss cells, and mix gently; (2) Add the cell plasmid mixture into the small groove of the electroporation cup, and cover the cap of the electroporation cup; ( 3) Set the program of the LONZA nuclear transfer instrument: select the V-024 program, insert the electro-transfer cuvette containing the cells and plasmids into the slot of the nuclear transfection instrument, and run the selected program; (4) After the program is finished, immediately ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com