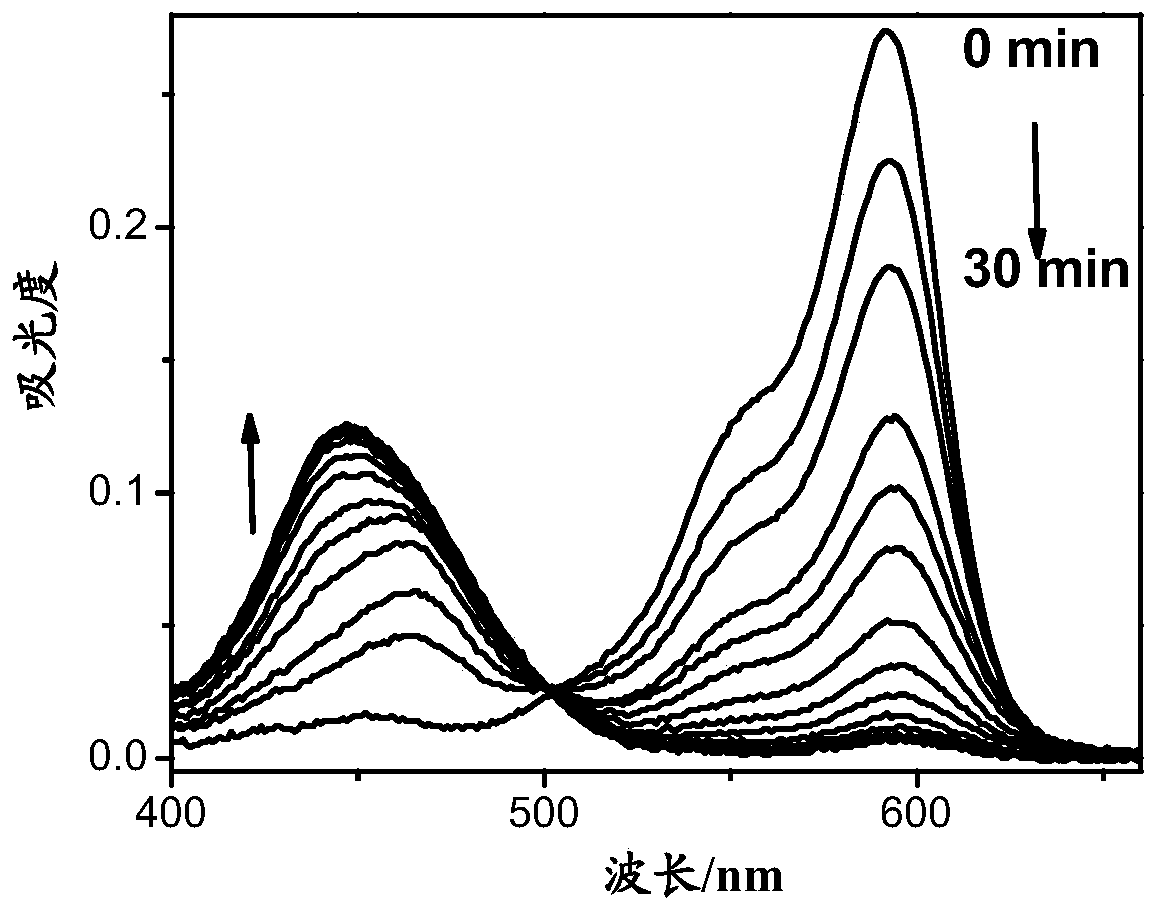
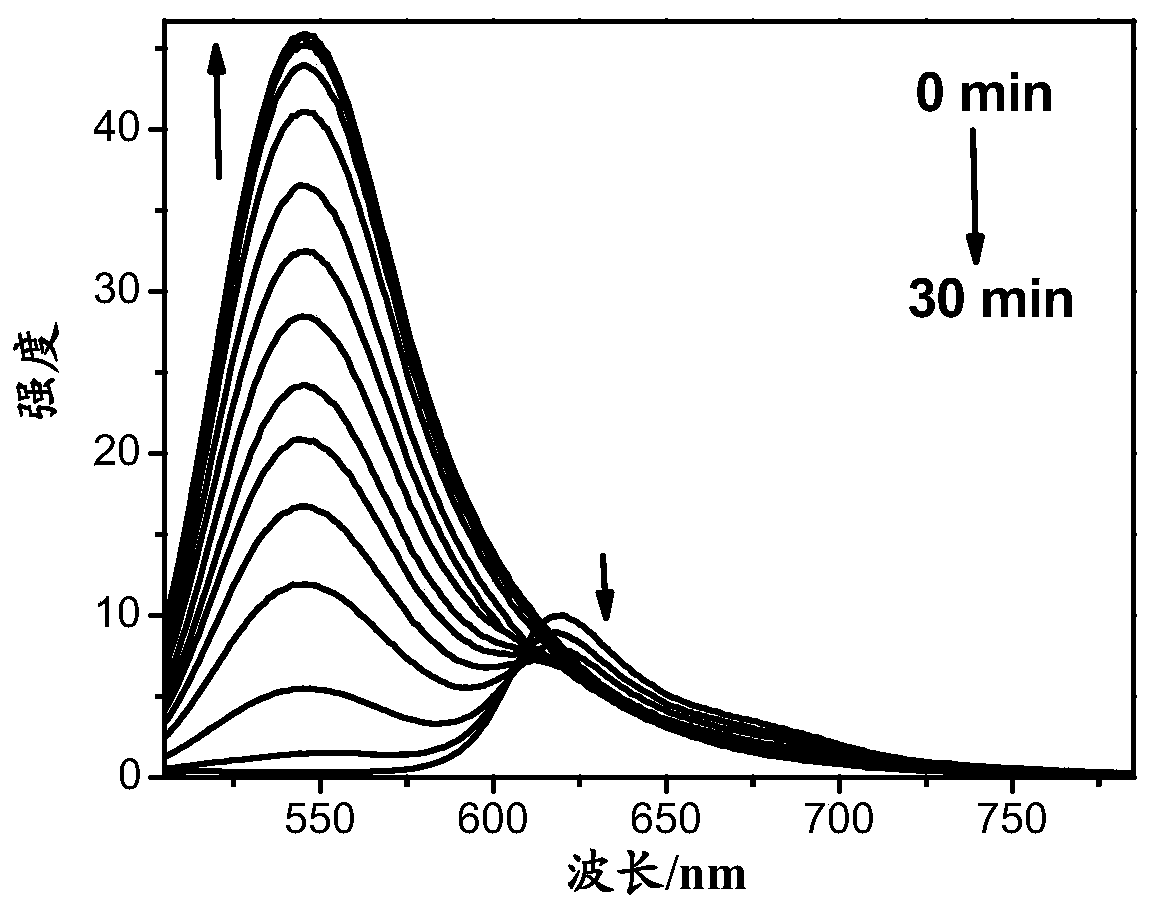
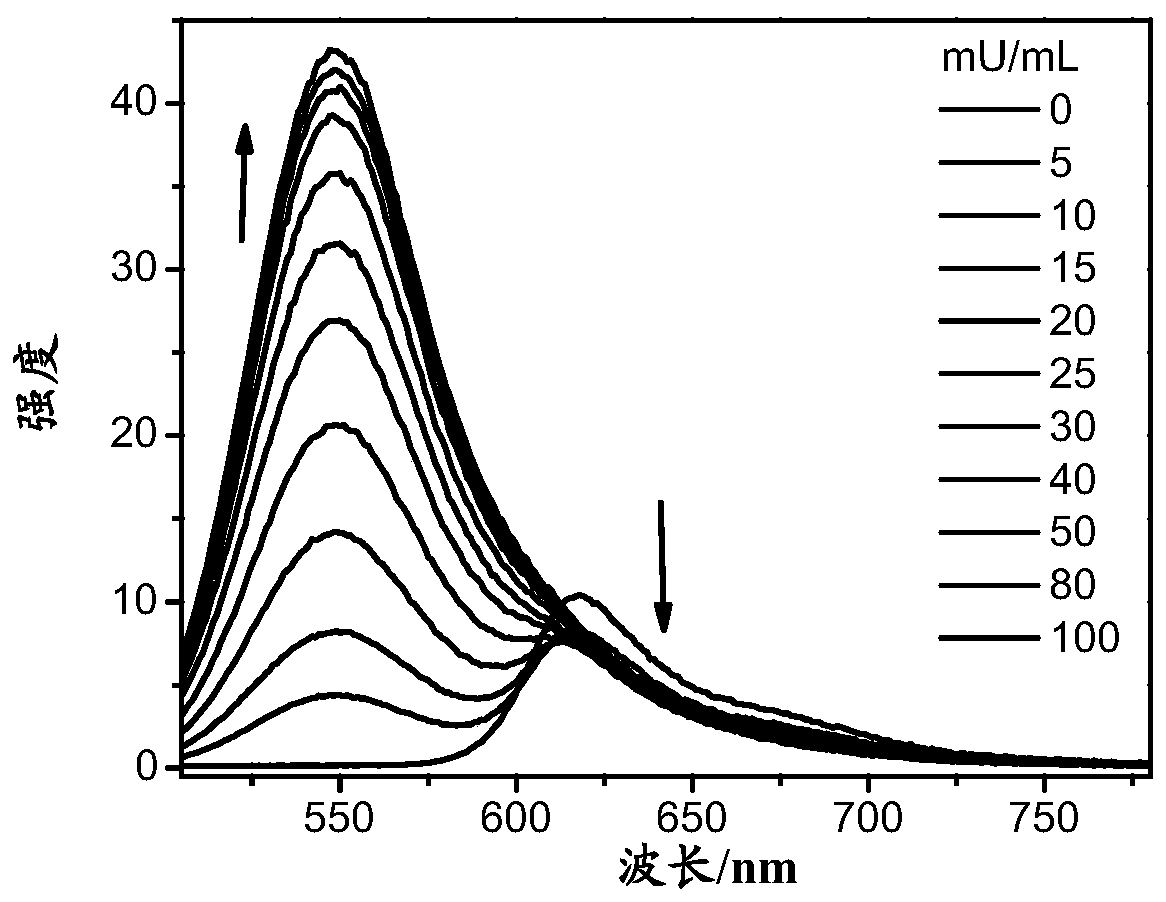
A compound, a product containing the compound and applications in detection of gamma-glutamyl transpeptidase
A glutamyl transpeptidase and compound technology, applied in the field of fluorescence detection, can solve the problems of low fluorescence ratio change times, slow response speed of fluorescent probes, detection of difficult tissue sample content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1, Preparation of γ-glutamyl transpeptidase-responsive fluorescent probe C3
[0166]
[0167]Compound A3 (0.5 g) was dissolved in 20 ml of methanol solution, and an aqueous solution (10 ml) of B1 (0.35 g) was added thereto at room temperature, and the reaction was continued for 12 hours at room temperature. After the reaction, the solvent was removed by rotary evaporation, and the crude product was separated and purified by high performance liquid chromatography (acetonitrile / water=2 / 1) to obtain compound C1 (0.21 g, yield 28%). The structure is confirmed as follows: 1 H NMR (400MHz,D 2 O, δ) 7.77(d, J=9.6Hz, 2H), 6.87(dd, J=9.6Hz, J=2Hz, 2H), 6.46(d, J=2Hz, 2H), 4.31(dd, J=8Hz , J=4.8 Hz, 1H), 3.6-3.2(m, 12H), 2.3-2.05(m, 3H), 1.9-1.8(m, 12H).
Embodiment 2
[0168] Example 2, Preparation of γ-glutamyl transpeptidase-responsive fluorescent probe C5
[0169]
[0170] Compound A5 (0.46 g) was dissolved in 20 ml of methanol solution, and an aqueous solution (12 ml) of B1 (0.35 g) was added thereto at room temperature, and the reaction was continued for 12 hours at room temperature. After the reaction, the solvent was removed by rotary evaporation, and the crude product was separated and purified by high performance liquid chromatography (acetonitrile / water=3 / 2) to obtain compound C5 (0.1 g, yield 29%). The structure is confirmed as follows: 1 H NMR (400MHz,D 2 O,δ)8.37(d,J=9.0Hz,1H), 8.29(d,J=15Hz,1H),8.26(d,J=9.0Hz,1H),8.06(d,J=15Hz,1H), 7.84 (t, J = 8.3Hz, 1H), 7.76 (m, 2H), 6.95 (dd, J1 = 2.4Hz, J2 = 9.6Hz, 1H), 6.69 (d, J = 2.2 Hz, 1H), 4.22 ( s, 3H), 4.03(m, 1H), 3.6-3.2(m, 9H), 2.06(t, 2H), 1.9-1.7(m, 2H), 1.32(d, J=7.2Hz, 3H).
Embodiment 3
[0171] Example 3, Preparation of γ-glutamyl transpeptidase-responsive fluorescent probe C7
[0172]
[0173] Compound A7 (0.4 g) was dissolved in 20 ml of methanol solution, and an aqueous solution (8 ml) of B1 (0.45 g) was added thereto at room temperature, and the reaction was continued for 24 hours at room temperature. After the reaction, the solvent was removed by rotary evaporation, and the crude product was separated and purified by high performance liquid chromatography (acetonitrile / water=4 / 1) to obtain compound C7 (0.1 g, yield 23%). The structure is confirmed as follows: 1 H NMR (400MHz,D 2 O,δ)7.28(d,J=9.6Hz,1H), 6.77(d,J=9.6Hz,1H),4.04(m,1H),3.6-3.2(m,3H),2.05(t,2H) ,1.9-1.8(m,2H), 1.32(d,J=7.2Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com