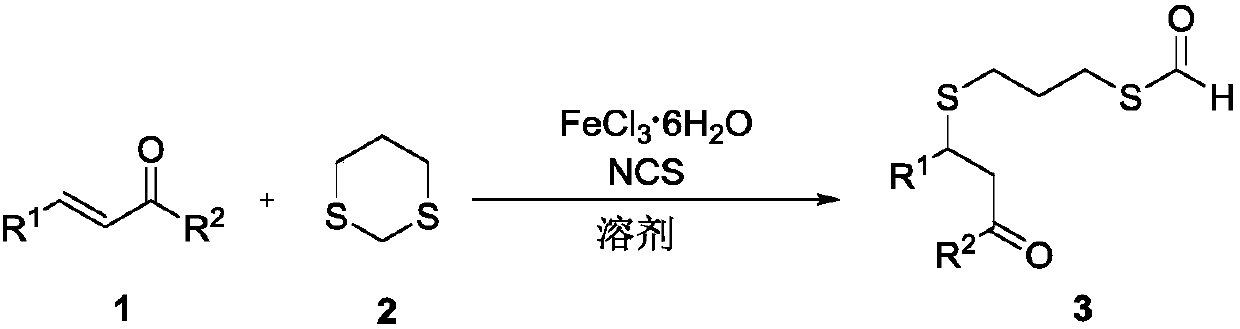
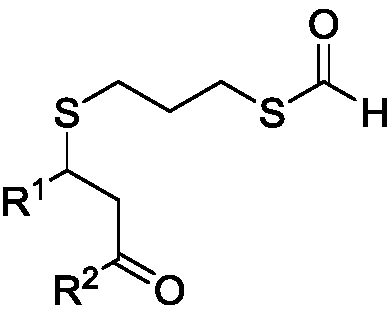
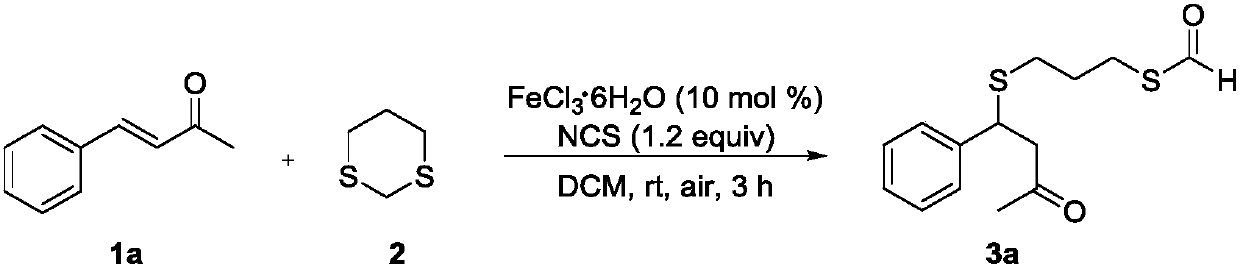
Method for preparing thioether-containing formyl sulpholipid conjugate
A technology containing thioether formyl thioester and formyl thioester, which is applied in the direction of organic chemistry, can solve the problems of large environmental pollution, highly toxic catalysts, and volatile toxicity of mercaptans, and achieves mild reaction conditions and cheap and easy reagents. The effect of easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012]
[0013] In a 10 ml round bottom flask, add 1,3-dithiane 2 (30 mg, 0.25 mmol), dissolve it with 2 ml of dichloromethane and add N-chlorosuccinimide (40 mg, 0.3 mmol), then Add FeCl 3 .6H 2 O (7mg, 0.025mmol), 1a (30mg, 0.21mmol), placed in air and stirred at room temperature for 3h. TLC detected that the reaction was stopped after the reaction was complete, and the product 3a was obtained by column chromatography after the solvent was evaporated. The resulting product data are characterized as follows: yellow oil; yield 89%; R f =0.18(EA / PE=1:10); 1 HNMR (300MHz, CDCl 3 )δ10.08(s,1H),7.42–7.15(m,5H),4.31(t,J=7.2Hz,1H),3.14–2.81(m,4H),2.51–2.25(m,2H),2.09 (s,3H),1.81–1.72(m,2H); 13 C NMR (75MHz, CDCl 3 )δ205.2, 187.4, 141.6, 128.6, 127.7, 127.4, 77.4, 77.0, 76.6, 49.9, 43.9, 30.7, 29.9, 28.7, 25.4; HRMS (ESI): m / z: calcd for C 14 h 19 o 2 S 2 [M+H] + :283.0826,found:283.0816.
Embodiment 2
[0015]
[0016] In a 10 ml round bottom flask, add 1,3-dithiane 2 (30 mg, 0.25 mmol), dissolve it with 2 ml of 1,2-dichloroethane and add N-chlorosuccinimide (40 mg, 0.3mmol), then add FeCl 3 .6H 2 O (7mg, 0.025mmol), 1b (34mg, 0.21mmol), placed in air and stirred at room temperature for 5h. TLC detected that the reaction was stopped after the reaction was complete, and the product 3b was obtained by column chromatography after the solvent was evaporated. The resulting product data are characterized as follows: yellow oil; yield 80%; R f =0.2(EA / PE=1:10); 1 HNMR (300MHz, CDCl 3 )δ10.08(s,1H),7.24–7.09(m,3H),7.06(s,1H),4.28(t,J=7.2Hz,1H),2.99–2.89(m,4H),2.42–2.29 (m,5H),2.10(s,3H),1.82–1.73(m,2H); 13 C NMR (75MHz, CDCl 3 )δ205.4, 187.5, 141.3, 138.2, 128.4, 128.2, 128.1, 124.6, 77.4, 77.0, 76.6, 49.8, 43.7, 30.7, 29.8, 28.6, 25.3, 21.4; HRMS (ESI): m / z: calcd for C 15 h 21 o 2 S 2 [M+H] + :297.0983,found:297.0980.
Embodiment 3
[0018]
[0019] In a 10 ml round bottom flask, add 1,3-dithiane 2 (30 mg, 0.25 mmol), dissolve it with 2 ml of dichloromethane and add N-chlorosuccinimide (40 mg, 0.3 mmol), then Add FeCl 3 .6H 2 O (7mg, 0.025mmol), 1c (38mg, 0.21mmol), placed in the air and stirred at room temperature for 8h. TLC detected that the reaction was stopped after the reaction was complete, and the product 3c was obtained by column chromatography after the solvent was evaporated. The resulting product data are characterized as follows: yellow oil; yield 83%; R f =0.11(EA / PE=1:10); 1 HNMR (300MHz, CDCl 3 )δ10.09(s,1H),7.29(d,J=0.9Hz,4H),4.30(t,J=7.2Hz,1H),3.05–2.89(m,4H),2.41–2.30(m,2H ),2.09(s,3H),1.83–1.73(m,2H); 13 C NMR (75MHz, CDCl 3 ))δ204.7, 187.2, 140.2, 133.0, 129.0, 128.7, 77.4, 77.0, 76.6, 49.8, 43.2, 30.6, 29.9, 28.7, 25.3; HRMS (ESI): m / z: calcd for C 14 h 18 ClO 2 S 2 [M+H] + :317.0437,found:317.0436.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com