Heteropolyvanadate compound and preparation method thereof
A heteropolyvanadate compound and crystallization technology, which is applied in organic chemistry methods, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc., can solve difficult problems of drug resistance, side effects of chemotherapy drugs, Expensive and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of a heteropolyvanadate compound
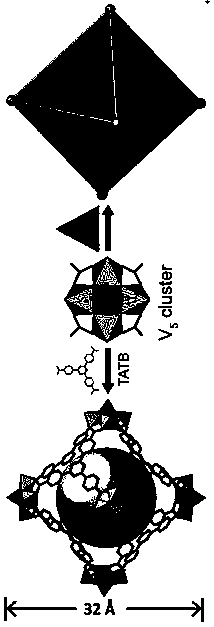
[0023] VCl 4 (20 mg, 0.104 mmol) and organic ligand 4,4,4-triazine-2,4,6-tribenzoic acid (abbreviated as: H 3 TATB, 25 mg, 0.057 mmol) was dissolved in 2 mL N,N-dimethylformamide (DMF) and 0.5 mL acetonitrile solution, stirred at room temperature for 30 minutes, and then transferred to a polytetrafluoroethylene reactor for crystallization at 130 °C for 48 hour, after the program was cooled to room temperature, the green crystals were isolated, washed with DMF, and dried at room temperature to obtain the target product (yield 69%, based on H 3 TATB); molecular formula [NH 2 (CH 3 ) 2 ] 12 [(V 5 o 9 Cl) 6 (TATB) 8 ].
Embodiment 2
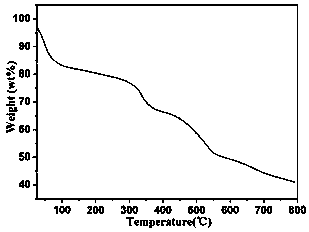
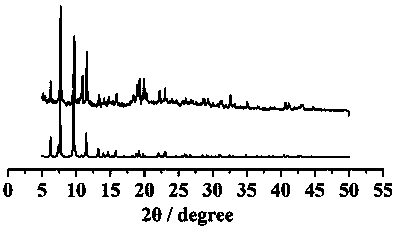
[0024] Example 2 Structural Analysis and Basic Characterization of Compounds
[0025] Compound [NH 2 (CH 3 ) 2 ] 12 [(V 5 o 9 Cl) 6 (TATB) 8 ] crystallized in the cubic crystal system, the space group is Fm-3m ,Cell parameters a = b = c =40.0473(9)Å, α=β=γ=90°, V=64227(4)Å 3 . The compound is a nanomolecular cage structure with an octahedral configuration, and each vertex of the octahedron is composed of [V 5 o 9 Cl]-built pentanuclear vanadyl clusters, each vanadyl cluster is bridged by four TATB organic ligands through terminal oxygen, and each TATB organic ligand is bridged by three carboxyl groups to three [V 5 o 9 Cl] clusters, each nanomolecular cage consists of six [V 5 o 9 Cl] clusters are bridged by eight organic ligands, TATB, to form a nanomolecular cage with an octahedral configuration. The size of the nanomolecular cage is 3.2 nm. Adjacent nanomolecular cages form a cubic packing pattern in space, forming a three-dimensional supramolecular comp...
Embodiment 3
[0031] Example 3 In vitro experiments to detect the inhibitory effect of compounds on tumor cells
[0032] 1. Experimental materials
[0033] Tumor cells: SMMC-7721 (human liver cancer cells)
[0034] MCF-7 (human breast cancer cells)
[0035] A549 (human lung cancer cells)
[0036] HL-60 (human leukemia cells);
[0037]Culture medium: RPMI640 medium containing 10% calf serum (medium for culturing SMMC-7721 cells), DMEM medium containing 10% calf serum (medium for cultivating MCF-7 and A549 cells), containing 10% IMEM medium (medium for culturing HL-60 cells) with calf serum.
[0038] 2. Experimental method
[0039] Resuscitate the cells by conventional methods, and adjust the cell concentration to 4×10 4 Cells / ml (SMMC-7721 cells, MCF-7 cells), 8×104 cells / ml (A549 cells), 1.5×105 cells / ml (HL-60 cells); 96-well plate culture, add cell suspension to each well 100 μl was cultured at 37°C and 5% CO2 for 24 hours, and then the culture solution of the corresponding cells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com