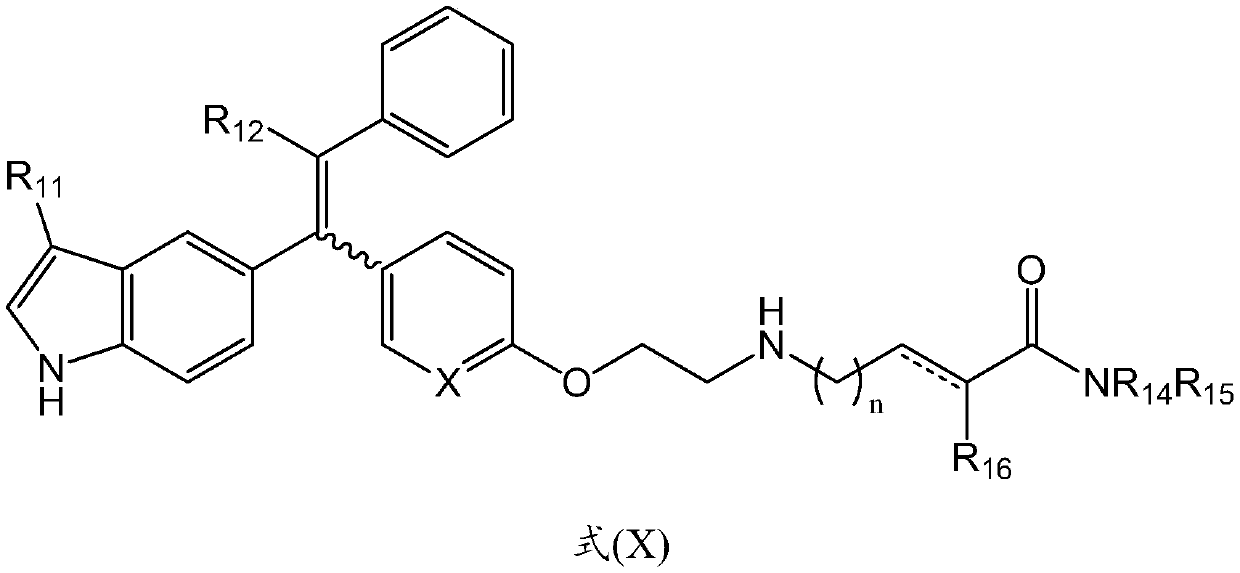
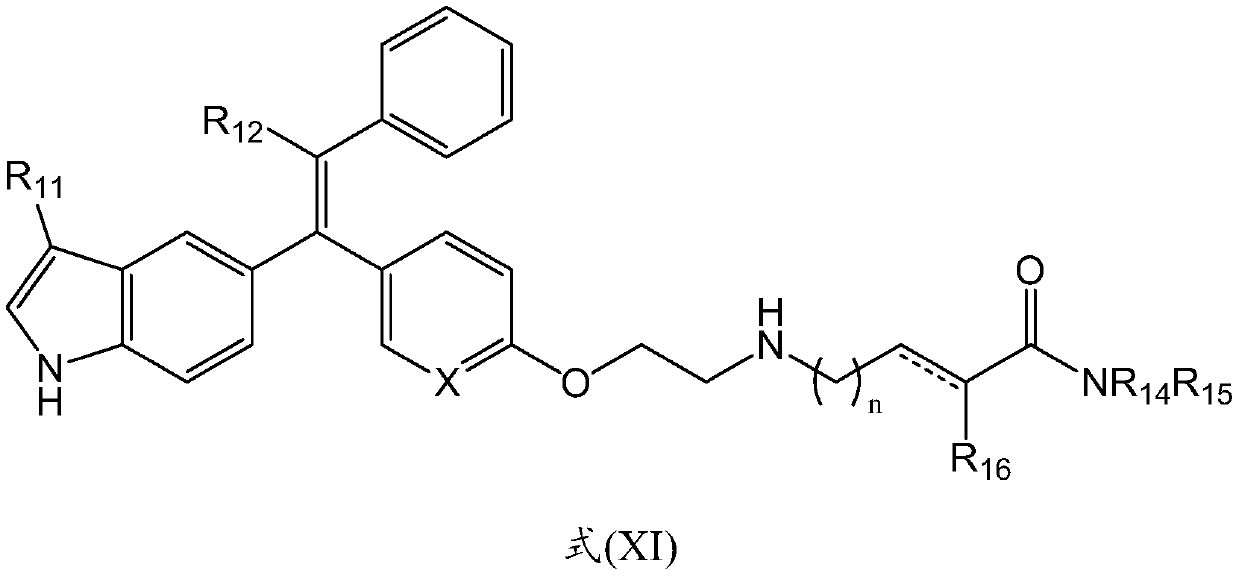
Tetrasubstituted alkene compounds and their use
An alkenyl and enamide technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as decreased bone density and increased risk of endometrial cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
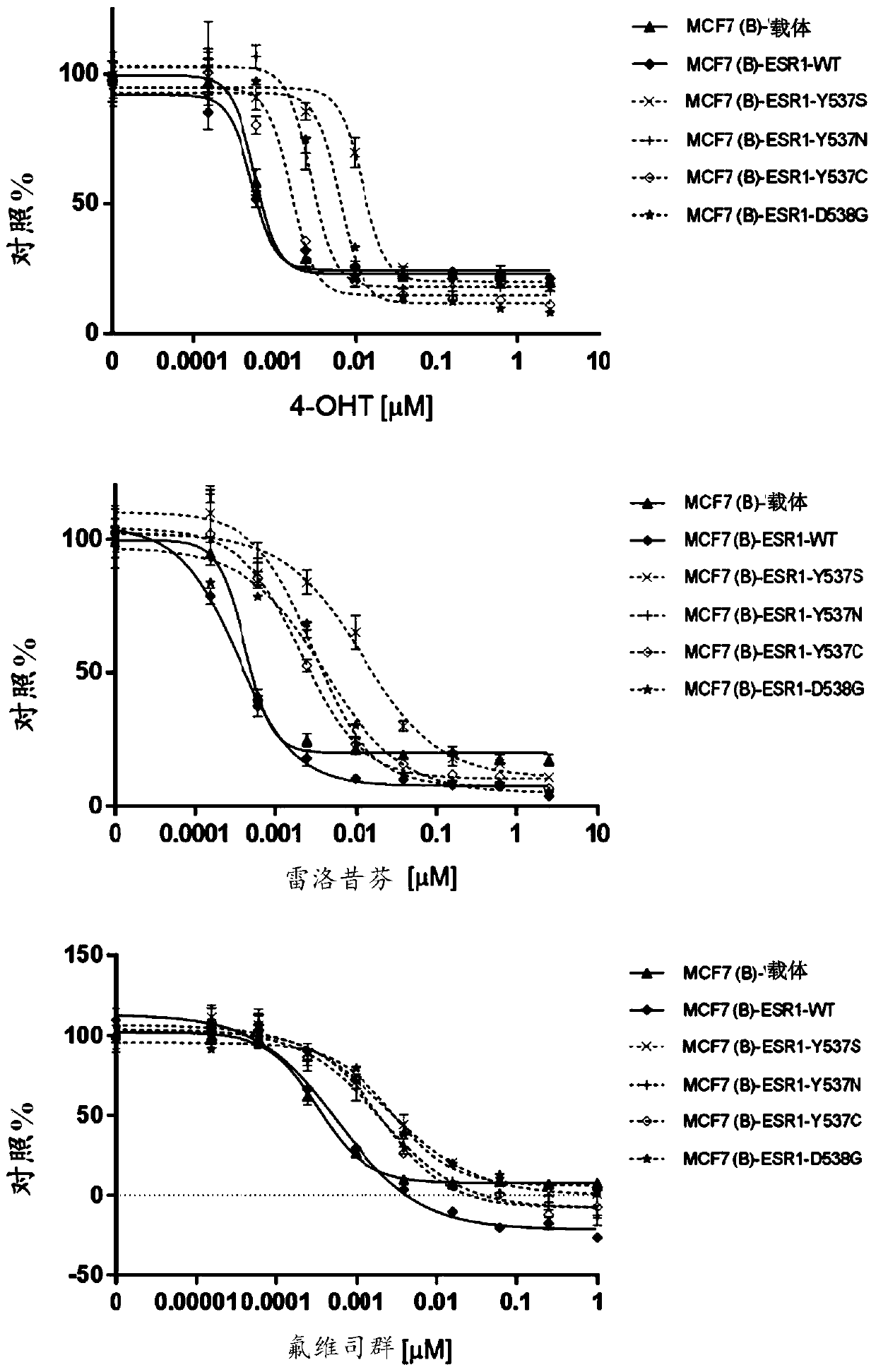
[0226] Non-limiting examples of embodiments of the compounds disclosed herein are provided herein. If there is any discrepancy between the depicted chemical structure of a compound and its chemical name, the depicted chemical structure shall prevail.
[0227] Table 1: Compounds
[0228]
[0229]
[0230]
[0231]
[0232]
[0233]
[0234]
[0235]
[0236]
Embodiment 201
[0238]
[0239] General procedure
[0240] The following abbreviations may be used in this document:
[0241] ACN: Acetonitrile
[0242] BOC: tert-butoxycarbonyl
[0243] CAN: Ammonium cerium nitrate
[0244] Conc.: concentrated
[0245] Cs 2 CO 3 : cesium carbonate
[0246] DABCO: 1,4-diazabicyclo[2.2.2]octane
[0247] DCM: dichloromethane
[0248] DHP: dihydropyran
[0249] DIPEA: N,N-Diisopropylethylamine, Hunig's base
[0250] DMA: Dimethylacetamide
[0251] DMF: Dimethylformamide
[0252] DMSO: Dimethylsulfoxide
[0253] DPEphos: (oxydi-2,1-phenylene)bis(diphenylphosphine)
[0254] EDCI·HCl: N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride
[0255] EtOH: ethanol
[0256] EtOAc: ethyl acetate
[0257] Et 3 N: Triethylamine
[0258] Ex.: Example
[0259] h: hours
[0260] HATU: 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridine 3-oxide hexafluorophosphate
[0261] HCl: hydrochloric acid
[0262] HMPA: Hexamethylphosphora...
Embodiment 101
[0292] Example 101: (2E)-N,N-Dimethyl-4-[[2-([5-[(1E)-4,4,4-trifluoro-1-(1H-indole-5- Synthesis of -1-phenylbut-1-en-2-yl]pyridin-2-yl]oxy)ethyl]amino]but-2-enamide (compound 101)
[0293]
[0294] Step 1: Synthesis of 5-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-1H-indole
[0295]
[0296] Into a 500 mL 3-neck round bottom flask purged and maintained with a nitrogen inert atmosphere was placed 5-bromo-1H-indole (15 g, 76.51 mmol, 1.00 equiv), THF (200 mL). Sodium hydride (4 g, 166.67 mmol, 1.30 equiv) was added portionwise at 0°C after this. Thereto was added SEMCl (14.05 g, 92.01 mmol, 1.10 equiv) dropwise at 0°C with stirring. The resulting solution was stirred at 25°C for 16 hours. The reaction mixture was cooled with a water / ice bath and cooled by adding 50 mL of NH 4 Quenched with Cl (sat aq). The resulting solution was extracted with 3 x 200 mL of ethyl acetate and the organic layers were combined and dried over anhydrous sodium sulfate and concentrated under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com