Production method of labelled vitamin D2 internal standard compound
A technology for internal standard compounds and vitamins, which can be applied in the fields of isotope introduction into organic compounds, organic chemistry methods, compounds of elements in Group 4/14 of the periodic table, etc., and can solve problems such as low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
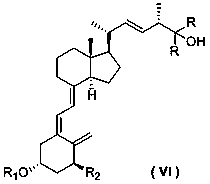
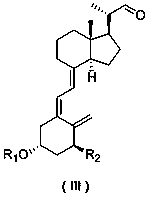
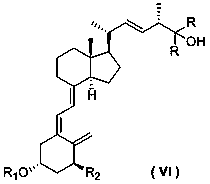
[0037] Add the following formula (IV-1) amide phosphorus salt compound (1.10 g, 2 mmol) into diethyl ether (10 mL), replace with nitrogen, start stirring, control the temperature at -20°C, and slowly add n-butyllithium in n-hexane (2.5M, 1.6 mL, 4 mol), the solution turned red. After the dropwise addition, the temperature was raised to room temperature, and the reaction was stirred for 2 hours; then the reaction solution was cooled to -20°C, and the following formula (Ⅲ) aldehyde (0.89 g , 2 mmol) solution in diethyl ether, the red color of the solution disappears, the reaction is stirred at constant temperature for 2 hours, warmed up to room temperature and stirred overnight; the reaction is quenched by adding dilute hydrochloric acid, the organic phase is separated, the aqueous phase is extracted with diethyl ether, the organic phase is combined, and anhydrous sulfuric acid Dry over magnesium; concentrate the organic phase, and purify by silica gel column chromatography (petr...
Embodiment 2
[0043] Add the following formula (IV-1) amide phosphorus salt compound (1.10 g, 2 mmol) into THF (10 mL), replace with nitrogen, start stirring, control the temperature at -20°C, and slowly add n-butyllithium in n-hexane solution (2.5M, 1.6 mL, 4 mol), the solution turned red. After the dropwise addition, the temperature was raised to 0°C, and the reaction was stirred for 2 hours; then the reaction solution was cooled to -20°C, and the aldehyde of the following formula (Ⅲ-1) was slowly added (0.89 g, 2 mmol) solution in THF, the red color of the solution disappeared, stirred at constant temperature for 2 hours, warmed up to room temperature and stirred overnight; quenched the reaction by adding dilute hydrochloric acid, extracted twice with ether, combined the organic phases, and dried over anhydrous magnesium sulfate ; Concentrate the organic phase and purify by silica gel column chromatography (petroleum ether / ethyl acetate: 5 / 1) to obtain 0.62 g of an intermediate of the fol...
Embodiment 3
[0051] Add the following formula (IV-1) amide phosphorus salt compound (1.10 g, 2 mmol) into THF (10 mL), replace with nitrogen, start stirring, control the temperature at -20°C, and slowly add n-butyllithium in n-hexane solution (2.5M, 1.6 mL, 4 mol), the solution turned red. After the dropwise addition, the temperature was raised to 0°C, and the reaction was stirred for 2 hours; then the reaction solution was cooled to -20°C, and the aldehyde of the following formula (Ⅲ-1) was slowly added (0.89 g, 2 mmol) solution in THF, the red color of the solution disappeared, stirred at constant temperature for 2 hours, warmed up to room temperature and stirred overnight; quenched the reaction by adding dilute hydrochloric acid, extracted twice with ether, combined the organic phases, and dried over anhydrous magnesium sulfate ; Concentrate the organic phase and purify by silica gel column chromatography (petroleum ether / ethyl acetate: 5 / 1) to obtain 0.62 g of the intermediate of the fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com