Method for detecting 1-phenyl-2-(N-pyrrolidinyl)-1-butanone
A pyrrolidinyl and phenyl technology, which is applied in the detection field of 1-phenyl-2--1-butanone, and can solve the problems of lack of new psychoactive substances and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
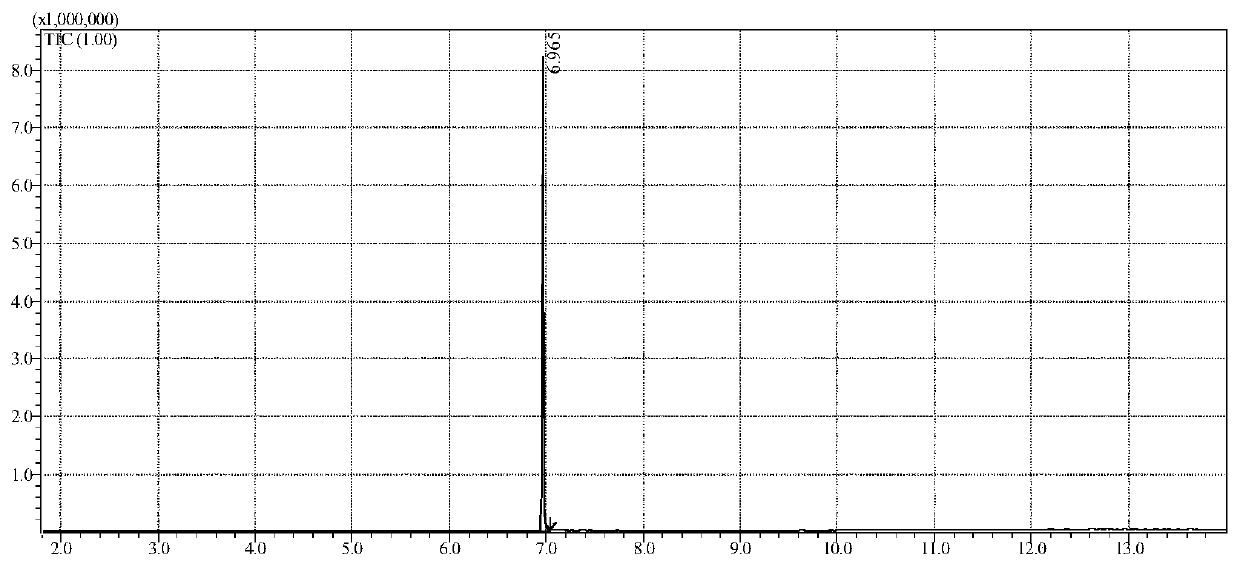
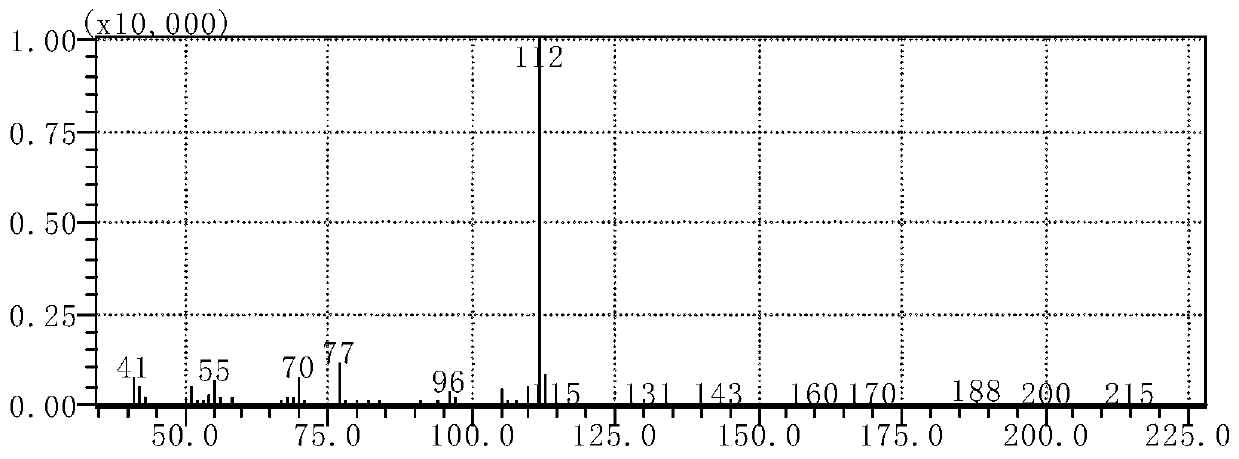
[0093] Example 1. Detection of 1-phenyl-2-(N-pyrrolidinyl)-1-butanone in suspected drug samples by gas chromatography-mass spectrometry and gas chromatography
[0094] 1 Reagents and standard substances, instruments and measuring instruments
[0095] 1.1 Reagents and standard substances
[0096] All reagents used in this method are of analytical grade.
[0097] Extraction reagent: Methanol.
[0098] Traceable reference material: α-PBP.
[0099] 1.2 Instruments and measuring instruments
[0100] Gas Chromatograph (GC) equipped with Flame Ionization Detector (FID).
[0101] Gas chromatography-mass spectrometry (GC-MS), a quadrupole mass spectrometer equipped with a standard electron impact (EI) source.
[0102] centrifuge.
[0103] Electronic balance, actual division value d=0.01mg.
[0104] oscillator.
[0105] 10mL and 1mL pipettes or pipettes.
[0106] 10mL bottle top pipette.
[0107] Note: All instruments and measuring instruments involved in this standard must pa...
Embodiment 2
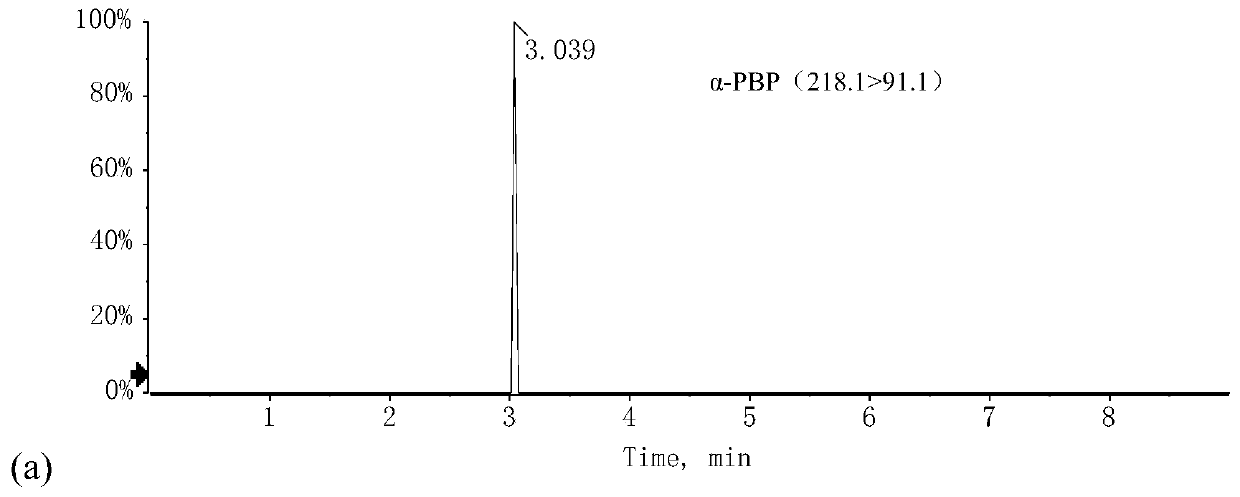
[0211] Example 2. Detection of 1-phenyl-2-(N-pyrrolidinyl)-1-butanone in suspected drug samples by liquid chromatography-tandem mass spectrometry and liquid chromatography
[0212] 1 Reagents and standard substances, instruments and measuring instruments
[0213] 1.1 Reagents and standard substances
[0214] The reagents used are chromatographically pure, and the test water is first-grade water (see GB / T 6682-2008). The reagents and standard substances include:
[0215] a) Reagents: methanol, acetonitrile, formic acid, phosphoric acid, triethylamine;
[0216] b) Traceable reference material: α-PBP;
[0217]c) 0.1% formic acid aqueous solution: measure 1mL of formic acid, add water to dissolve and dilute to 1000mL, mix well, filter through a 0.22μm filter membrane, and let it stand for use after ultrasonication;
[0218] d) Phosphoric acid-triethylamine buffer solution: Measure 4.12mL of concentrated phosphoric acid, add 200mL of water to dilute; measure 5.56mL of triethylam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Volume fraction | aaaaa | aaaaa |
| Volume fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com