A method of separating and/or extracting lanthanides
A technology of lanthanide and separation coefficient, applied in the field of extraction and separation of lanthanide in water, can solve the problems of poor selectivity, narrow acid and alkali resistance range, poor separation ability of radioactive ions, etc., and achieve the effect of high selective removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 [V 3 o 7 ] n n- Preparation of layered framework materials
[0071] Mix the vanadium source, lithium hydroxide, N,N-dimethylformamide solution and water evenly in a certain molar ratio, stir thoroughly at room temperature, and put it into a stainless steel reaction kettle lined with polytetrafluoroethylene. After reacting at constant temperature for a period of time, naturally cool to room temperature, filter the obtained sample, wash with distilled water and ethanol in turn, and dry naturally to obtain [Me 2 NH 2 ] V 3 o 7 (Sample 1#). The kind of reactant, reactant ratio, reaction time, reaction temperature, productive rate are shown in Table 1 in detail.
[0072] Table 1
[0073]
[0074] Yield = (mass of obtained sample 1#) ÷ (mole number of vanadium in the vanadium source ÷ 2 x theoretical molecular weight of the product) x 100%.
[0075] The concentration of the N,N dimethylformamide solution can be adjusted as required, eg 40wt%.
Embodiment 2
[0076] Example 2 utilizes sample 1# to extract and separate the kinetic test of lanthanide elements
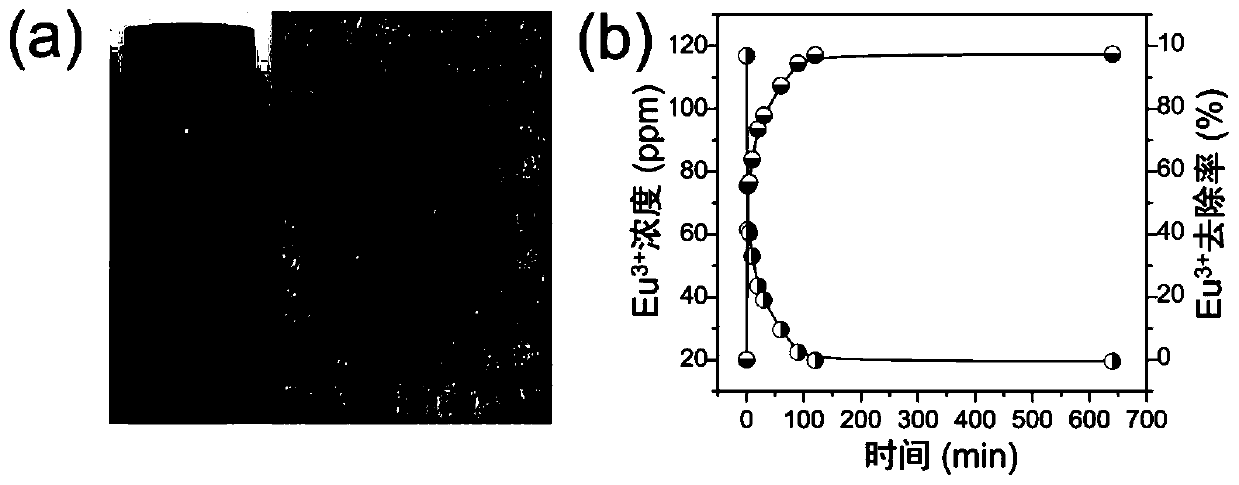
[0077] After grinding, the powder of sample 1# and 116.9ppm of Eu 3+ The aqueous solution of ions is mixed, and the mixture is stirred at 50° C., V (volume of solution): m (mass of exchanger) = 1000 mL / g. Take a small amount of mixture supernatant at regular intervals, and use inductively coupled plasma emission spectrometry to determine Eu 3+ ion concentration. figure 1 The picture in (a) is a photo of sample 1#; figure 1 In (b) is Eu with time 3+ Changes in ion concentration and Eu 3+ Ion removal rate graph. figure 1 (b) shows that sample 1# has a good effect on Eu 3+ The removal of ions can reach equilibrium within 2 hours.
Embodiment 3
[0078] Example 3 Utilize sample 1# to extract and separate the adsorption model test of lanthanide elements
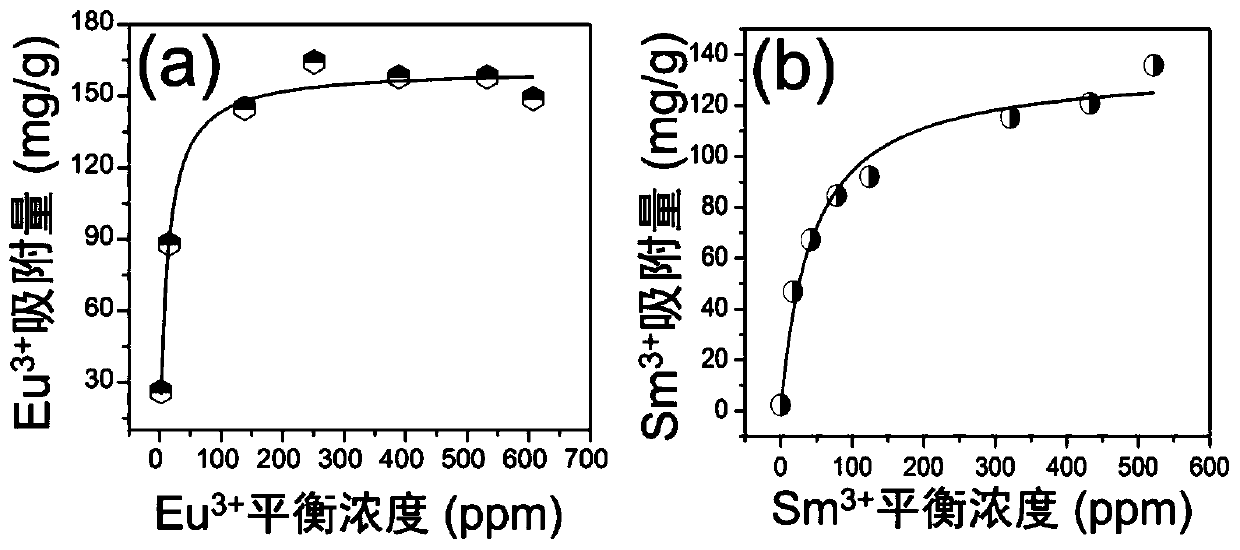
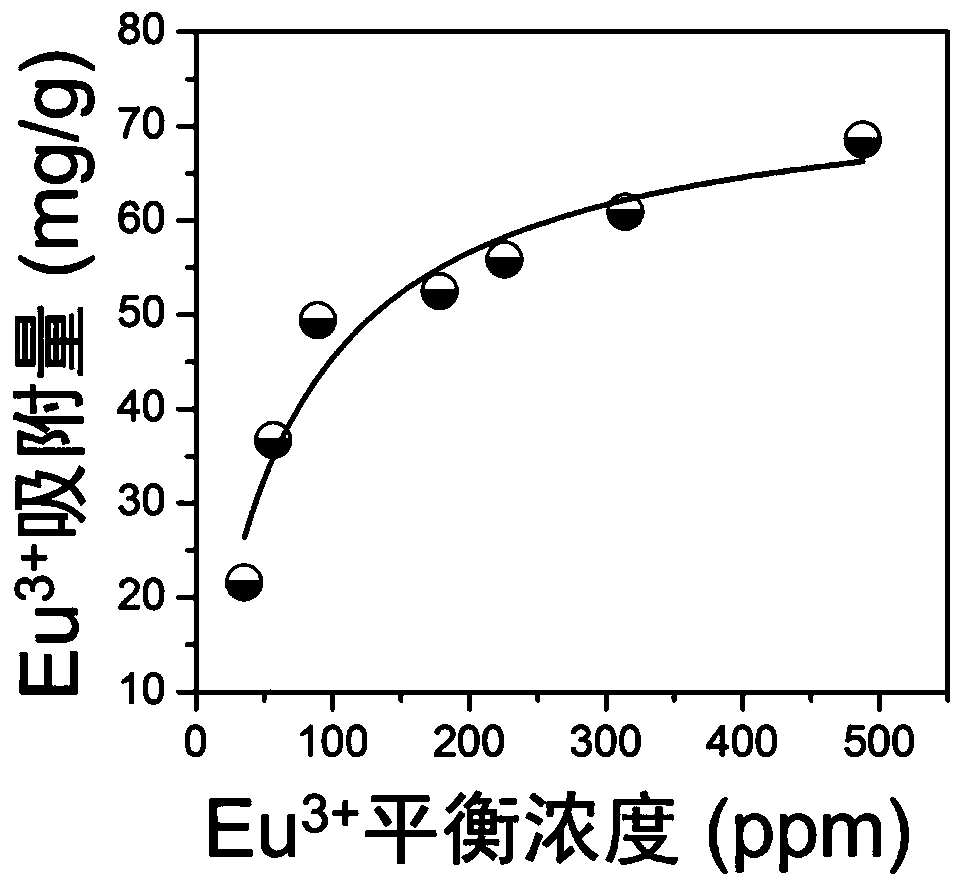
[0079] After grinding, the powder of sample 1# was mixed with 2.2-756.2ppm Eu 3+ 、Sm 3+ The ionic water solution was mixed, and the mixture was stirred at 50° C. for 3 h, V (solution volume): m (mass of exchanger) = 1000 mL / g. After the reaction was completed, the supernatant and the starting solution were taken to measure Eu by inductively coupled plasma emission spectrometry. 3+ 、Sm 3+ concentration of ions. Experimental results such as figure 2 , figure 2 (a) and (b) respectively show the effect of sample 1# on Eu 3+ 、Sm 3+ The maximum adsorption capacity of ions can reach 161 mg / g and 135 mg / g respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com