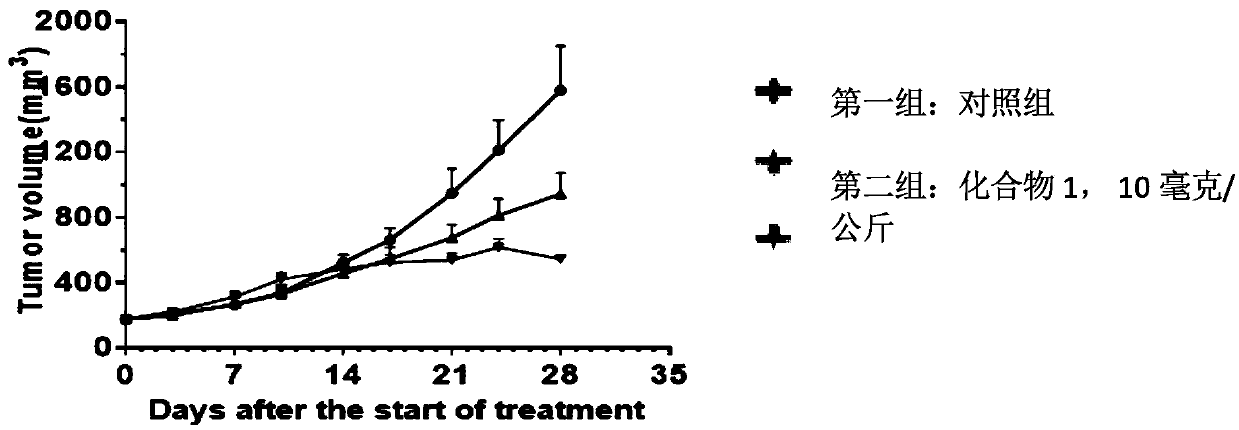
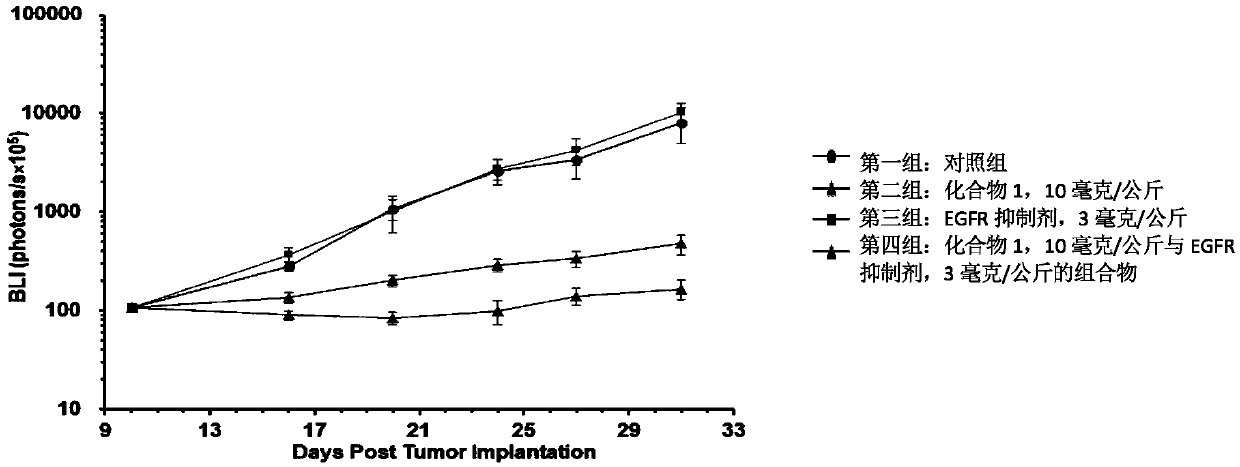
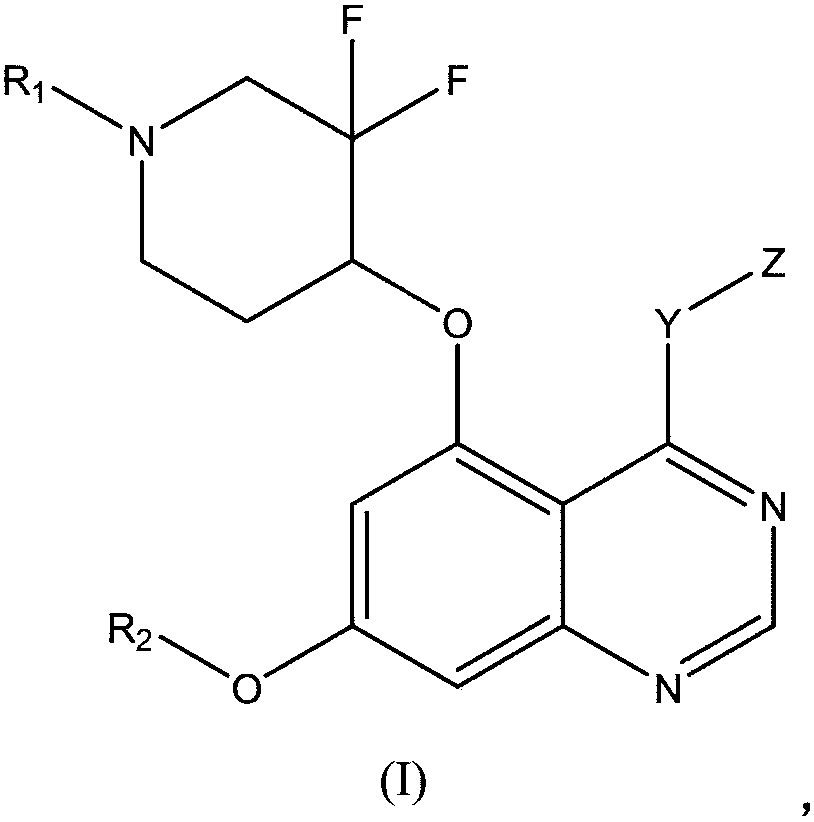
5-substituted difluoro piperidine compound capable of penetrating blood brain barrier
A compound and drug technology, applied in the field of hydrates and their polymorphs, 5-substituted difluoropiperidine derivatives, and its salts, can solve problems such as limited therapeutic effects, reduce drug resistance, and improve tablet intake compliance Sexuality, good pharmacokinetics and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0310] The synthesis of intermediate 2,6-difluoro-4-deuteromethoxybenzonitrile A2, the synthetic route is as follows:
[0311]
[0312] Step: Add deuterated methanol (698 mg, 19.34 mmol), azodicarbonyldipiperidine (4.88 g, 19.34 mmol) and tributylphosphine (3.91 g, 19.34 mmol). The reaction mixture was stirred at 65 °C for 3 hours and cooled to room temperature. The reaction mixture was filtered, concentrated, and purified by silica gel column (n-hexane / ethyl acetate=20:1) to obtain product A2 (500 mg, 45% yield) as a pale yellow solid. LC-MS: (ESI) m / z=173 (M+H) + .
[0313] Synthesis of intermediate 2,6-difluoro-4-ethoxybenzonitrile A3, the synthetic route is as follows:
[0314]
[0315] To a solution of A1 (7.0 g, 45.2 mmol) in N,N-dimethylformamide (100 mL) was added iodoethane (14 g, 90.3 mmol) and potassium carbonate (12.46 g, 90.3 mmol). The reaction mixture was stirred at 60 °C for 2 hours and cooled to room temperature. After the reaction mixture was conc...
Embodiment 2
[0334] Synthesis of compounds 1, 17 and 33: ((R / S)-5-((3,3-difluoro-1-methylpiperidin-4-yl)oxy)-4-((4-fluoro- Synthesis of 2-methyl-1H-indol-5-yl)oxy)-7-methoxyquinazoline (1) and separation into enantiomerically pure (R)- 5-((3,3-difluoro-1-methylpiperidin-4-yl)oxy)-4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy )-7-methoxyquinazoline 1(17) and (S)-5-((3,3-difluoro-1-methylpiperidin-4 base)oxy)-4-((4- Fluoro-2-methyl-1H-indol-5-yl)oxy)-7-methoxyquinazoline 1(33), the synthetic route is as follows:
[0335]
[0336] Step 1: Add 4-fluoro-2-methyl-1H-indol-5-ol (15 mg, 0.091 mmol) and cesium carbonate (30 mg , 0.091 mmol) was stirred at 70°C for 1 hour under nitrogen protection, cooled to room temperature, added water (20 mL), and extracted with ethyl acetate (20 mL, 3 times). The organic phase was dried over sodium sulfate and concentrated in vacuo to give a residue, which was purified by silica gel column (dichloromethane / methanol=300:1 to 100:1) to give the product 1 as a whit...
Embodiment 3
[0339]Synthesis of compounds 4, 20 and 36: (R / S)-N-(5-chlorobenzo[d][1,3]dioxol-4-yl)-5-((3,3 -Synthesis of -difluoro-1-methylpiperidin-4-yl)oxy)-7-methoxyquinazolin-4-amine (4) and separation into enantiomerically pure (R)-N-(5-chlorobenzo[d][1,3]dioxol-4-yl)-5-((3,3-difluoro-1-methylpiperidine -4-yl)oxy)-7-methoxyquinazolin-4-amine (20) and (S)-N-(5-chlorobenzo[d][1,3]dioxolane En-4-yl)-5-((3,3-difluoro-1-methylpiperidin-4-yl)oxy)-7-methoxyquinazolin-4-amine (36), synthesized The route is as follows:
[0340]
[0341] Step 1: A mixture of A7 (223 mg, 0.568 mmol) and 5-chlorobenzo[d][1,3]dioxol-4-amine (217 mg, 1.137 mmol) in acetic acid (20 mL) , stirred at 80°C for 16 hours under nitrogen protection. After cooling, it was treated with saturated sodium bicarbonate solution to pH = 8 and extracted with dichloromethane. The organic phase was dried over sodium sulfate and concentrated in vacuo to give a residue, which was purified by silica gel column (dichloromethane / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com